Corvida Wagler, 1830
At this point the passerines divide into two branches, the Corvida and Passerida. Corvida is the smaller branch, comprised of about 835 species. Since there are many more species in Passerida, we place Corvida first. The use of “Corvida” for the clade sister to Passerida follows the logical terminology of Cracraft et al. (2004), although the term Corvoidea, or even “core Corvoidea” is in common use. Recently, Corvides (and Passerides) seem to be gaining ground following their use in H&M-4 (Dickinson and Christidis, 2014). The actual membership of Corvida is a bit different from Cracraft et al. (2004) as some families have been moved eleswhere in the phylogeny.
Current thinking is that the Corvida have their origin in Australia/New Guinea (the actual continent of Australia). In particular, their diversity may have been developed during the late Eocene/early Oligocene in the New Guinea archipelago, and then certain taxa pumped out into Asia and the rest of the world. I particularly commend to your attention the discussion in Jønsson et al. (2011b) and secondarily in Aggerbeck et al. (2014). Where they overlap, I consider the dates in Jønsson et al. (2016) more reasonable.
Recent Taxonomic History and Updates
The taxonomy in Barker et al. (2004) suggested Corvida is divided into two superfamilies, Callaeoidea and Corvoidea. However, Irestedt and Ohlson (2009) argued that the families in Barker et al.'s Callaeoidea (Melanocharitidae, Cnemophilidae, Callaeidae, and by implication, Notiomystidae) are really basal Passerida. They show that if the analysis is done without the RAG-1 gene, these families end up in Passerida. Apparently RAG-1 has a strong signal that overwhelms conflicting evidence from other genes. Since the RAG-1 signal is not supported by other genes, they argue it is spurious. Although there is room for dispute here, I found Irestedt and Ohlson's argument sufficiently convincing to follow it here. More recent analyses such as Aggerbeck et al. (2014) and Jønsson et al. (2011b) and Moyle et al. (2016) also support removing these taxa from Corvida.
In late 2013 I restructured the Corvida based on Aggerbeck et al. (2014). They examined up to 22 nuclear loci, and were able to create a generally well-supported phylogeny for Corvida. However, the thin taxon sampling means that some of that support may be less than it seems. Previously, the most comprehensive study of the Corvida was the six-gene analysis by Jønsson et al. (2011b), which includes one of more representative from each of the corvid families except the monotypic Pityriaseidae (bristlehead). Most of the well-supported portions of Jønsson et al.'s (2011b) phylogeny are pretty much the same as Aggerbeck et al. However, Aggerbeck et al. were able to find good support for much more of their phylogeny.
Another restructing came in December 2015 following the publications by Marki et al. (2015) and Jønsson et al. (2016). These two papers, with 5 overlapping co-authors both use a similar data set. Marki et al. used up to 7 nuclear and 4 mitochondrial genes, while Jønsson et al. added an eighth nuclear gene (fib5). Indeed, Jønsson et al. sampled a large fraction (86%) of the corvid taxa, far beyond what anyone else has done. In comparison, Aggerbeck et al. (2014) only covered about 5% of the corvids. I have ended up with a tree that is a compromise between Aggerbeck et al. (2014) and Jønsson et al (2016). The tree in Marki et al. (2015) will get limited use here as it is only sparsely labeled.
Well, here it is 2017 and Corvid taxonomy remains contentious. Moyle et al.'s (2016) latest research has complicated it further. They use a different method, focusing on ultraconserved elements. They assembled a dataset averages almost 4300 ultraconserved elements for 104 songbird species, including taxa from many of the corvid families. Although their results are broadly similar to previous studies, there are several important differences. Attempts to form a consensus tree didn't pan out and I have decided to follow their results whenever possible. This excepts the Corvoidea, where their coverage was rather sparse and where they had conflicting results concerning the relation of birds-of-paradise, crows, and monarchs.
Changes include returning Campephagidae (cuckooshrikes) to a more basal position and separating the Cinclosomatidae (quail-thrushes and jewel-babblers) from the Falcunculidae (shriketits), in spite of strong support for a close relationship in both Aggerbeck et al. (2014) and Jønsson et al. (2016). Moyle et al. (2016) also pair the Neosittidae (sitellas) and Mohouidae (whitehead and allies). I'm not convinced this last pairing is not just long branch attraction, but am following it for now. They also support a sister relationship between Pachycephalidae (whistlers) and Oriolidae (orioles) as in Jønsson et al., but not Aggerbeck et al. The regrouping in Orioloidea has caused me to move the whistlers and orioles to the end of Orioloidea. There are some other differences involving minor families: Eulacestomaidae (ploughbill), Psophodidae (whipbirds), and the exact placement of Machaerirhynchidae (boatbills) and Rhagologidae (mottled berryhunter, formerly whistler).
The Corvoidea have not been touched by the latest reorganization and there are still some issues in this group. The first is the relation between the drongos (Dicruridae) and the fantail clade (Lamproliidae/Rhipiduridae). It's clear enough that these are the basal taxa in Corvoidea, but in what order? Aggerbeck et al. (2014) tell us that the fantails are basal. Marki et al. (2015) have the drongos basal. Finally, Jønsson et al. (2016) consider them sisters. Given the ambiguity (and short internodes), I'm treating them as equally basal, listing the drongos first as the smaller group.
The next issue is the monarchs. These too are troublesome, with three papers giving three different answers. Aggerbeck et al. put them in a clade with the birds-of-paradise (Paradisaeidae). Marki et al. have them sisters to the jays and crows (Corvidae). J&soslash;nsson et al. put them sister to a shrike/crow clade. Moyle et al. (2016) gave two solutions: one with the monarchs basal to the birds-of-paradise and mudnesters and the shrikes and crows, the other with them nearer the birds-of-paradise, and the mudnesters, shrikes, and crows grouped together. I've ended up with the shrikes and crows together (unlike Marki et al.), and have put the monarchs on their own branch, between the clades containing the birds-of-paradise and the crows. Also, I don't really trust the positions of the mudnesters, melampittas, or ifrita.
The TiF Corvida Tree
Several superfamilies are marked on the TiF corvid tree shown below. According to Moyle et al. (2016), all of them date to about 19-22 mya. Jønsson et al. date them somewhat earlier, in the mid-Oligocene, roughly 28 million years ago (I think the dates in Aggerbeck et al. are too old).
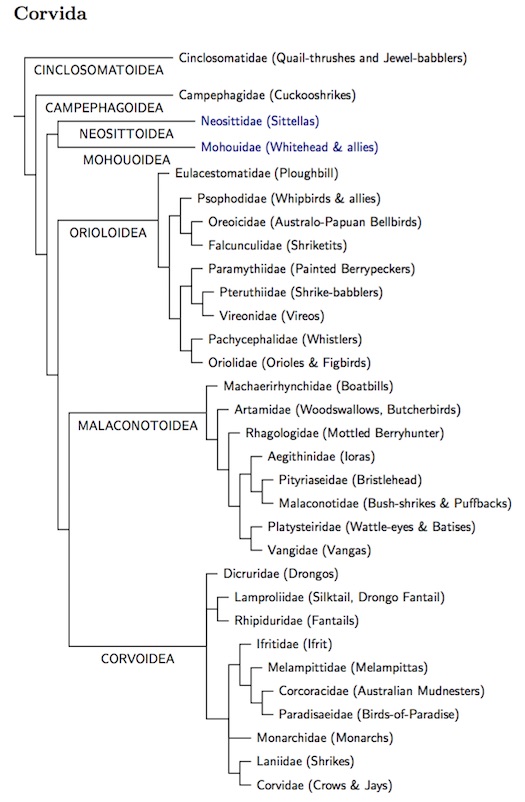
Cinclosomatoidea Mathews, 1921-2
The Cinclosomatidae are an Australo-Papuan family. I've placed them in their own superfamily based on Moyle et al. (2016), although both Aggerback et al. (2014) and Jønsson et al. (2016) have them allied with Falcunculidae with strong support, a grouping that never made much sense to me. Moyle et al. estimate the Cinclosomatoidea branch dates to about 22 mya.
Cinclosomatidae: Quail-thrushes and Jewel-babblers Mathews, 1921-2
2 genera, 12 species HBW-12 in Eupetidae (pp. 365-8)
 |
| Cinclosomatidae species tree |
|---|
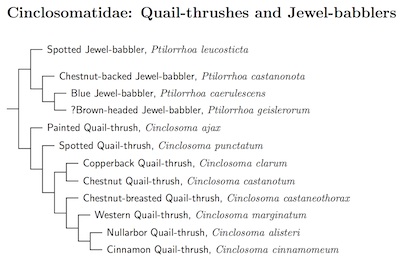
Toon et al. (2012) examined DNA from a number of Quail-thrushes. The arrangement of Cinclosoma is based on their work. They found that at least two subspecies should be regarded as full species. This involves splitting Western Quail-thrush, Cinclosoma marginatum from Chestnut-breasted Quail-thrush, Cinclosoma castaneothorax (both now monotypic) and Nullarbor Quail-thrush, Cinclosoma alisteri, from Cinnamon Quail-thrush, Cinclosoma cinnamomeum (including tirariense).
Chestnut Quail-thrush / Chestnut-backed Quail-thrush, Cinclosoma castanotum, is split into Copperback Quail-thrush, Cinclosoma clarum, and Chestnut Quail-thrush, Cinclosoma castanotum, based on Dolman and Joseph (2015).
- Spotted Jewel-babbler, Ptilorrhoa leucosticta
- Chestnut-backed Jewel-babbler, Ptilorrhoa castanonota
- Blue Jewel-babbler, Ptilorrhoa caerulescens
- Brown-headed Jewel-babbler, Ptilorrhoa geislerorum
- Painted Quail-thrush, Cinclosoma ajax
- Spotted Quail-thrush, Cinclosoma punctatum
- Copperback Quail-thrush, Cinclosoma clarum
- Chestnut Quail-thrush Quail-thrush, Cinclosoma castanotum
- Chestnut-breasted Quail-thrush, Cinclosoma castaneothorax
- Western Quail-thrush, Cinclosoma marginatum
- Nullarbor Quail-thrush, Cinclosoma alisteri
- Cinnamon Quail-thrush, Cinclosoma cinnamomeum
Campephagoidea Vigors, 1825
The Campephagoidea are the next branch according to Moyle et al. (2016). That makes them the first large branch in Corvida. However, Aggerback et al. (2014), Jønsson et al. (2016), and Marki et al. (2015) found them sister to Malaconotoidea. Again we are dealing with short internodes where accurate placement can be a problem. Moyle et al. date the divergence of Campephagoidea to about 20 mya. As is generally the case, both Aggerbeck et al. and Jønsson et al. prefer an earlier dating, about 27 mya.
Campephagidae: Cuckooshrikes Vigors, 1825
12 genera, 93 species HBW-10
Although Australo-Papua is the ancestral region for the Corvida, the Campephagidae have expanded well beyond that. They are widespread in the southern portions of the Old World (Afrotropics, Indomalaya, Australasia), and are even present in a limited area of the eastern Palearctic.
 |
| Click for Campephagidae species tree |
|---|
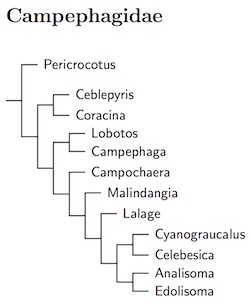
The ordering of the minivets (Pericrocotus) is based on Jønsson et al. (2010b). The results in Fuchs et al. (2007a) and Jønsson et al. (2008a, b) suggested some generic boundaries need to be redrawn for Coracina and related genera. Jønsson et al. (2010c) analyzed most of the revelant taxa, and the current organization is based on their results. Five species of Coracina have been separated as Ceblepyris (Cuvier 1816, type cinereus). See H&M-4 (Dickinson and Christidis, 2014) and Jønsson et al. (2010c, 2016).
They found that several of the Coracina cuckooshrikes were actually closer to some of the trillers (Lalage). They advocate use of a broad genus Lalage including the species listed here in Malindangia, Cyanograucalus, Celebesica, Analisoma, and Edolisoma. The extra genera here serve to highlight the major groups among Lalage and allies. McGregor's Cuckooshrike, Malindangia mcgregori, is basal among them. The rest are in two clades. One includes the species listed below in Lalage. Further division of this did not seem warranted. The other clade probably includes the rest of the Jønsson et al.'s broad Lalage.
The support for grouping Cyanograucalus (Hartlaub, 1861) and Celebesica (Strand, 1928, replacing the preoccupied Celebesia Riley, 1918) is relatively low, so I've put them in separate genera. Indeed, there is some chance they don't even group with the rest of Lalage. I've separated the three Analisoma (Mathews, 1928) because the cicadabirds (Edolisoma, Jacquinot and Pucheran, 1853) form a tight group that is relatively distant from the Analisoma.
The Admiralty Islands Cicadabird, Edolisoma admiralitatis, has been split from Common Cicadabird, Edolisoma tenuirostre (H&M 4; Jønsson et al., 2010c).
There is a deep division (ca. 21 mya) between the minivets and cuckooshrikes. This is recognized by placing the minivets in a separate subfamily, Pericrocotinae.
Pericrocotinae: Minivets Sundevall, 1872
- White-bellied Minivet, Pericrocotus erythropygius
- Jerdon's Minivet, Pericrocotus albifrons
- Small Minivet, Pericrocotus cinnamomeus
- Fiery Minivet, Pericrocotus igneus
- Gray-chinned Minivet, Pericrocotus solaris
- Sunda Minivet, Pericrocotus miniatus
- Short-billed Minivet, Pericrocotus brevirostris
- Little Minivet, Pericrocotus lansbergei
- Long-tailed Minivet, Pericrocotus ethologus
- Orange Minivet, Pericrocotus flammeus
- Scarlet Minivet, Pericrocotus speciosus
- Ryukyu Minivet, Pericrocotus tegimae
- Ashy Minivet, Pericrocotus divaricatus
- Rosy Minivet, Pericrocotus roseus
- Swinhoe's Minivet, Pericrocotus cantonensis
Campephaginae: Cuckooshrikes Vigors, 1825
- Madagascan Cuckooshrike, Ceblepyris cinereus
- Comoros Cuckooshrike, Ceblepyris cucullatus
- Grauer's Cuckooshrike, Ceblepyris graueri
- Gray Cuckooshrike, Ceblepyris caesius
- White-breasted Cuckooshrike, Ceblepyris pectoralis
- Stout-billed Cuckooshrike, Coracina caeruleogrisea
- Hooded Cuckooshrike, Coracina longicauda
- Cerulean Cuckooshrike, Coracina temminckii
- Ground Cuckooshrike, Coracina maxima
- Pied Cuckooshrike, Coracina bicolor
- Barred Cuckooshrike, Coracina lineata
- Boyer's Cuckooshrike, Coracina boyeri
- Black-faced Cuckooshrike, Coracina novaehollandiae
- North Melanesian Cuckooshrike, Coracina welchmani
- White-bellied Cuckooshrike, Coracina papuensis
- Manus Cuckooshrike, Coracina ingens
- Javan Cuckooshrike, Coracina javensis
- Wallacean Cuckooshrike, Coracina personata
- Buru Cuckooshrike, Coracina fortis
- Moluccan Cuckooshrike, Coracina atriceps
- South Melanesian Cuckooshrike, Coracina caledonica
- Large Cuckooshrike, Coracina macei
- Bar-bellied Cuckooshrike, Coracina striata
- Andaman Cuckooshrike, Coracina dobsoni
- Sunda Cuckooshrike, Coracina larvata
- Slaty Cuckooshrike, Coracina schistacea
- White-rumped Cuckooshrike, Coracina leucopygia
- Western Wattled-Cuckooshrike, Lobotos lobatus
- Eastern Wattled-Cuckooshrike, Lobotos oriolinus
- Black Cuckooshrike, Campephaga flava
- Red-shouldered Cuckooshrike, Campephaga phoenicea
- Petit's Cuckooshrike, Campephaga petiti
- Purple-throated Cuckooshrike, Campephaga quiscalina
- Golden Cuckooshrike, Campochaera sloetii
- McGregor's Cuckooshrike, Malindangia mcgregori
- Polynesian Triller, Lalage maculosa
- Samoan Triller, Lalage sharpei
- Long-tailed Triller, Lalage leucopyga
- White-shouldered Triller, Lalage sueurii
- White-winged Triller, Lalage tricolor
- Rufous-bellied Triller, Lalage aurea
- Black-browed Triller, Lalage atrovirens
- White-browed Triller, Lalage moesta
- Varied Triller, Lalage leucomela
- Mussau Triller, Lalage conjuncta
- Black-and-white Triller, Lalage melanoleuca
- Pied Triller, Lalage nigra
- White-rumped Triller, Lalage leucopygialis
- Mauritius Cuckooshrike, Lalage typica
- Reunion Cuckooshrike, Lalage newtoni
- Black-winged Cuckooshrike, Lalage melaschistos
- Black-headed Cuckooshrike, Lalage melanoptera
- Indochinese Cuckooshrike, Lalage polioptera
- Lesser Cuckooshrike, Lalage fimbriata
- Blue Cuckooshrike, Cyanograucalus azureus
- Pygmy Cuckooshrike, Celebesica abbotti
- Halmahera Cuckooshrike, Celebesica parvula
- New Caledonian Cuckooshrike, Analisoma anale
- Blackish Cuckooshrike, Analisoma coerulescens
- White-winged Cuckooshrike, Analisoma ostentum
- Black-bellied Cuckooshrike, Edolisoma montanum
- Kai Cicadabird, Edolisoma dispar
- Pale-shouldered Cicadabird, Edolisoma dohertyi
- Pale Cicadabird, Edolisoma ceramense
- Black-bibbed Cicadabird, Edolisoma mindanense
- Admiralty Islands Cicadabird, Edolisoma admiralitatis
- Makira Cicadabird, Edolisoma salomonis
- Black-bellied Cicadabird / Solomons Cuckooshrike, Edolisoma holopolium
- Black-shouldered Cicadabird, Edolisoma incertum
- Gray-headed Cuckooshrike, Edolisoma schisticeps
- Black Cicadabird, Edolisoma melas
- Sulawesi Cicadabird, Edolisoma morio
- Gray-capped Cicadabird, Edolisoma remotum
- Sula Cicadabird, Edolisoma sula
- Common Cicadabird, Edolisoma tenuirostre
- Palau Cicadabird, Edolisoma monacha
- Yap Cicadabird, Edolisoma nesiotis
- Pohnpei Cicadabird, Edolisoma insperatum
Neosittoidea Ridgway, 1904
One common thread in most recent analyses of the Corvida is that the sittellas, which were formerly considered whistlers, end up on a branch by themselves. Jønsson (2008b) had the branch occur after the Corvoidea-Malaconotoidea split. Barker et al. (2004) considered them more basal in the Corvida. But they still end up on their own branch. Aggerbeck et al. (2014) have them sister to Eulacestoma, but even they find support for this to be somewhat weak. Most recently, Jønsson et al. (2016) put then basal to the rest of the Malaconotoidea, including Campephagidae. I'm following Moyle et al. (2016), who put them on a deep branch sister to Mohouidae. Moyle et al. estimate the split between Neosittoidea and Mohouoidea at around 19 mya, and the split between the two of the and the rest of Corvida only slightly earlier.
Neosittidae: Sittellas Ridgway, 1904
1 genus, 3 species HBW-12
The sittellas are nuthatch-like birds of Australia and New Guinea. Indeed, they were considered nuthatches as late as 1967. They aren't nuthatches, and aren't even members of Passerida. Like many Australasian birds, they are part of Corvida.
- Varied Sittella, Daphoenositta chrysoptera
- Papuan Sittella, Daphoenositta papuensis
- Black Sittella, Daphoenositta miranda
Mohouoidea Mathews, 1946
Jønsson et al. (2011b) was the first genetic study to suggest any real affinities for Mohouoidea (sister to Campephagidae), although the support for this was weak. Although Jønsson et al. (2011b) were unable to place Mohouoidea reliably, Aggerbeck et al. (2014) and Jønsson et al. (2016) found strong support for Mohouoidea as the basal clade in Corvida. Moyle et al. (2016) placed them together with Neosittiodea, and sister to the Orioloidea/Malaconotoidea/Corvoidea clade.
Mohouidae: Whitehead & allies Mathews, 1946
2 genera, 3 species Not HBW Family
The Mohouidae are New Zealand endemics that have previously been included in many different families. Writing in HBW-13, Boles (2007) mentions Paridae, Timaliidae, Orthonychidae, Campephagidae, Sylviidae, Maluridae, and Acanthizidae (=Pardalotidae). The Acanthizidae option was followed by Sibley and Monroe (1990), Dickinson et al. (2003) and version 2.0 of the IOC checklist (Jan 2009). Boles (2007) and HBW include them in Pachycephalidae. However, the evidence for any of these is very weak.
Boles merges Finschia into Mohoua. They appear to be fairly closely related, possibly congeneric, whether one looks at osteology (Olson, 1990) or DNA hybridization (Sibley and Ahlquist, 1987). However, Jønsson et al. (2016) estimated a common ancestor roughly 9-10 million years ago, so perhaps they are not quite so close after all. Regardless of whether they are placed in one genus or two, the Pipipi is basal, and the Whitehead and Yellowhead are sister to one another (Aidala et al., 2013).
- Pipipi, Finschia novaeseelandiae
- Whitehead, Mohoua albicilla
- Yellowhead, Mohoua ochrocephala
Orioloidea Vigors, 1825
 |
| Click for Orioloidea genus tree |
|---|
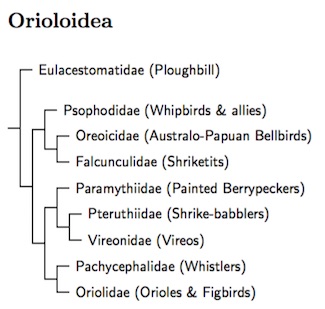
Orioloidea is the next large grouping in Corvida. I follow the Moyle et al. (2016) topology. The monotypic ploughbill family (Eulacestomatidae) is basal. Next, there is small group of three families: whipbirds and wedgebills (Psophodidae), Australo-Papuan bellbirds (Oreoicidae), and shriketits (Falcunculidae). The main part of Orioloidea has two large branches. The first contains the painted berrypeckers (Paramythiidae), shrike-babblers (Pteruthiidae, recently separated from the vireos), and the much bigger vireo family (Vireonidae). The second consists of the whistlers (Pachycephalidae) and orioles (Oriolidae).
There is disagreement about whether the sittellas (Neosittidae) belong here, with Aggerbeck et al. (2014) in favor and Jønsson et al. (2016) against. These two papers also disagree concerning the relationships of the familes in Orioloidea. Marki et al. (2015), using a dataset similar to Jønsson et al., gives an arrangement that is close to that of Aggerbeck et al. Moyle et al. (2016) is somewhat different, removing Cinclosomatidae entirely to the root of Corvida and placing Eulacestomatidae basal in Orioloidea. Like Jønsson et al., Moyle et al. have the whistlers and orioles as sister families.
Eulacestomatidae: Ploughbill Schodde and Christidis, 2014
1 genus, 1 species Not HBW Family (HBW-12:409)
The Ploughbill is a New Guinea endemic. HBW treated them as a basal whistlers, along with 6 other taxa that have also been removed from the whistlers. The others are Pachycare (Pardalotidae), Falcunculus (Falcunculidae), Oreoica and Aleadryas (Oreoicidae), Rhagologus (Rhagologidae), and Hylocitrea (Hypocoliidae).
Its closest relatives remain obscure. Aggerbeck et al. (2014) placed it sister to the sittellas. Other studies have come to different conclusions. Both Aggerbeck et al. (2014) and Jønsson et al. (2016) place it near a clade containing the painted berrypeckers, shrike-babblers, and vireos. Moyle et al. (2016) have it as the basal group in Orioloidea, as is done here.
- Wattled Ploughbill, Eulacestoma nigropectus
Psophodidae: Whipbirds and Wedgebills Bonaparte, 1854
3 genera, 6 species HBW-12 in Eupetidae (pp. 370-2)
This small group of Australo-Papuan birds has been substantially reduced in size. I have previously treated these taxa and other possibily related taxa such as Paramythiidae in various ways (e.g., based on Norman et al., 2009a). The current version is based on Aggerbeck et al. (2014) and Jønsson et al. (2016), leaving a very narrowly circumscribed Psophodidae.
Jønsson et al. include Psophodidae in this clade, but this is only weekly supported. Both Aggerbeck et al. (2014) and Marki et al. (2015) put Psophodidae near the vireos even though the Marki et al. dataset is quite similar to the Jønsson et al. dataset. I'm following Moyle et al., which puts Psophodidae here.
Other birds that have previously been considered to belong in this family include Cinclosoma and Ptilorrhoa (currently in Cinclosomatidae) and Eulacestoma, which is now in its own family. There is also some support for including Falcunculus (Falcunculidae), in Psophodidae (e.g, Barker et al., 2004; Dumbacher, 2008), but the multigene analyses of Jønsnon et al. (2016), Aggerbeck et al. (2014), Norman et al. (2009a) and Jønsson et al. (2011b) all separate them. Jønsnon et al. (2016) date each of these families as 23-25 million years old, while Moyle et al. (2016) date them in the 17-19 mya range.
Sibley and Monroe's Cinclosomatinae included two additional genera: Eupetes and Ifrita, while HBW-12's corresponding Eupetidae (del Hoyo et al., 2007) also includes Melampitta. Barker et. al (2004) suggested that Melampitta belonged in the monarchs (Monarchidae), but with weak support. They and the mudnesters (Corcoracidae) were thought to form a clade. Reddy and Cracraft (2007), using the same genes, found Melampitta either with the monarchs or mudnesters. Dumbacher et al. (2008) found it related to Ifrita, but did not include the necessary taxa to say where they goes. Irestedt et al. (2008) considered Melampitta basal in the narrowly construed Corvoidea, while Norman et al. (2009a) include Ifrita with the monarchs, as do Jønsson et al. (2016). Jønsson et al. (2011b) put Ifrita with the monarchs and Melampitta in a clade containing the mudnesters and birds-of-paradise. Aggerbeck et al. (2014) have the mudnesters, Ifrita and Melampitta in a clade with the birds-of-paradise. In any event, they do not belong here.
 |
| Psophodidae species tree |
|---|
Jønsson et al. (2007) showed that the rail-babbler Eupetes was really related to Chaetops and Picathartes. It is now in its own family Eupetidae within Passerida.
The Psophodidae have been rearranged and divided into three genera based on Toon et al. (2013) and Jønsson et al. (2016). The three genera are used because the divisions within the family are deeper than usually supposed. The genus Androphobus has been merged into Psophodes because Jønsson et al. (2016) found it is sister to the Eastern Whipbird. The Wedgebills take the genus name Sphenostoma (Gould 1838, type cristatum) and the Western and Mallee Whipbirds become genus Phodopses (Schodde and Mason 1999, type nigrogularis).
- Western Whipbird, Phodopses nigrogularis
- Mallee Whipbird, Phodopses leucogaster
- Chiming Wedgebill, Sphenostoma occidentale
- Chirruping Wedgebill, Sphenostoma cristatum
- Papuan Whipbird, Psophodes viridis
- Eastern Whipbird, Psophodes olivaceus
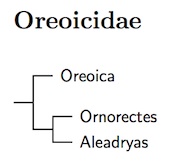
Oreoicidae: Australo-Papuan Bellbirds Sibley & Ahlquist, 1985
3 genera, 3 species Not HBW Family (HBW-12:411, 435)
 |
| Oreoicidae genus tree |
|---|
Because divisions between them are fairly deep, dating to near the Oligocene/Miocene boundary. Jønsson et al. (2016) estimate a common ancestor for the three families in the late Oligocene at about 26 million years. Moyle et al. (2016) put the divisions in the early Miocene, following the Wallacea uplift. Although the grouping of Falcunculidae and Cinclosomatidae was strongly supported in Aggerbeck et al. (2014) and Jønsson et al. (2016), support for including Oreoicidae was less strong. Moyle et al. (2016) have Cinclosomatidae basal in Corvida while Falcunculidae remains here, and that is the solution I follow.
There has support for other arrangements. Although both Aggerbeck et al. (2014) and Jønsson et al. (2016) both place Oreoicidae near Falcunculidae and Cinclosomatidae, earlier analyses based on fewer genes have come to different conclusions. Norman et al. (2009a) weakly supported placing Aleadryas and Oreoica in Malaconotoidea while Jønsson et al. (2008a) put Aleadryas and the former Pitohui, Ornorectes, near Campehagidae.
Jønsson et al. (2016) found the three Oreoicidae species to form a strongly-supported group. They even suggest putting them all in the same genus (Oreoica has priority), but with the split between them somewhere around 10 million years ago, I do not see a compelling reason to do this.
I accept the name Oreoicidae (Sibley and Ahlquist, 1985a) over Oreoicidae (Schodde and Christidis, 2014). Schodde and Christidis object that there is not a proper definition or description of Oreoicini Sibley and Ahlquist. However, they treat it as monotypic, and I think that defining the tribe Oreoicini as consisting of Crested Bellbird is sufficient definition.
- Crested Bellbird, Oreoica gutturalis
- Crested Pitohui, Ornorectes cristatus
- Rufous-naped Whistler, Aleadryas rufinucha
Falcunculidae: Shriketits Chenu and des Murs, 1853
1 genus, 3 species Not HBW Family (HBW-12:409)
There doesn't seem to be strong evidence for regarding the Falcunculidae as one species instead of three. I have a hunch the lineages are older than many think, so I treat them as three species.
- Northern Shriketit, Falcunculus whitei
- Western Shriketit, Falcunculus leucogaster
- Eastern Shriketit, Falcunculus frontatus
Paramythiidae: Painted Berrypeckers P. L. Sclater, 1893
2 genera, 2 species HBW-13
This family of New Guinea endemics was previously removed from the Melanocharitidae (Passerida) and placed in their own family in Corvida. It has been unclear whether they should be treated as a separate family, and the TiF list has sometimes included them in Psophodidae. Aggerbeck et al. (2014) found them to be a separate branch sister to Psophodidae and Vireonidae (presumably including Pteruthiidae). Jønsson et al. (2016) put them sister to the the Vireonidade and Pteruthiidae, about 26 million years distant from their closest relatives. Moyle et al. (2016) concur with Jønsson, but put the separateion at about 19 mya. The latter topology is followed here.
Sibley and Ahlquist (1987) removed the painted berrypeckers from Melanocharitidae (berrypeckers and longbills), but left them in place near the flowerpeckers and sunbirds (others had previously questioned the affinities of these species). See also Sibley and Ahlquist (1990) and Sibley and Monroe (1990). Nonetheless, H&M-3 (Dickinson, 2003) still had them united in a single family, but now inside what I call Corvida. They were separated again in Barker et al. (2004), this time with the Paramythiidae located near Oriolus (as they are) and with the Melanocharitidae as basal Corvida near Callaeatidae. As previously mentioned, Irestedt and Ohlson (2009) recognized that Melanocharitidae, Cnemophilidae, and Callaeidae, were really basal Passerida, and that the RAG-1 gene had given a misleading signal. Aggerbeck et al.'s 22-gene analysis (2014) supports this interpretion.
- Tit Berrypecker, Oreocharis arfaki
- Crested Berrypecker, Paramythia montium
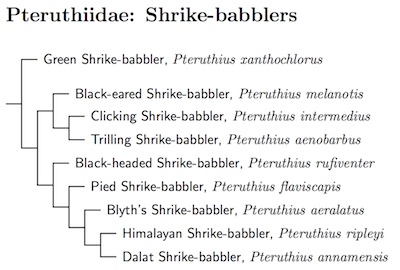
Pteruthiidae: Shrike-babblers Informal?
1 genus, 9 species Not HBW Family (HBW-12:284-6)
 |
| Pteruthiidae species tree |
|---|
I had previously treated this Indo-Malayan group as a subfamily of the vireos. They are fairly distantly related to the vireos, with a common ancestor about 23 million years ago according to Jønsson et al. (2016), but about 15 mya according to Moyle et al. (2016). This coincides with the emergence of New Guinea in the mid-Miocene and makes a lot of sense. Still, its enough to justify separate families which serve to highlight the geographic separation between the shrike-babblers and almost all of the vireos.
Reddy (2008), using the phylogenetic species concept, advocated splitting Pteruthius into 19 species. While this seems extreme, Reddy's evidence suggests that 5 species were too few. Rheindt and Eaton (2009) reexamined the issue taking into consideration plumage, morphology, and vocalizations. Combining this information with Reddy's genetic analysis, they suggest that Pteruthius contains 9 biological species. Their treatment is followed here.
- Green Shrike-babbler, Pteruthius xanthochlorus
- Black-eared Shrike-babbler, Pteruthius melanotis
- Clicking Shrike-babbler, Pteruthius intermedius
- Trilling Shrike-babbler, Pteruthius aenobarbus
- Black-headed Shrike-babbler, Pteruthius rufiventer
- Pied Shrike-babbler, Pteruthius flaviscapis
- Blyth's Shrike-babbler, Pteruthius aeralatus
- Himalayan Shrike-babbler, Pteruthius ripleyi
- Dalat Shrike-babbler, Pteruthius annamensis
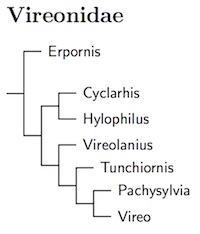
Vireonidae: Vireos Swainson, 1837
7 genera, 57 species HBW-15
Next come the vireos. They were once thought to be closely related to the wood-warblers (Parulidae). When Sibley and Ahlquist discovered the corvid group, they found that the vireos (and shrikes) were members of it. Some thought it odd that the vireos, with an old world origin, had no remaining old world members. It is true that it seemed odd. But it is false that there are no old world members.
The old world vireos and relatives have been hiding out among the babblers (Timaliidae), living in southeast Asia and the Himalayas. The first to be noticed was Erpornis zantholeuca (nee Yuhina zantholeuca). Cibois et al. (2002) discovered it was not a babbler, but belonged somewhere in the Corvida. Rather than being a babbler like the other Yuhinas, it is actually a vireo relative (Barker et al., 2004).
Reddy and Cracraft (2007) found that the shrike-babblers (Pteruthius) are not babblers at all, but are vireo relatives. I currently list them in their own family, sister to the vireos, but it would also be reasonable to treat them as a subfamily of the vireos.
Erporninae: Erpornis Informal?
- White-bellied Erpornis, Erpornis zantholeuca
Vireoninae: Vireos, Greenlets and allies Swainson, 1837
 |
| Click for Vireonidae species tree |
|---|
The current arrangment of the Vireoninae (what was traditionally considered the vireo family) is based on Slager et al. (2014) (see also the preliminary results posted by Battey, 2014). They sampled most of the taxa. The conventional Hylophilus turned out to be paraphyletic. To solve this, one species moves to Vireo (Tepui Greenlet, now Tepui Vireo, Vireo sclateri) and the remaining Hylophilus are split into three genera: Hylophilus (scrub greenlets), Tunchiornis (tawny-crowned greenlets, Slager and Klicka, 2014b), and Pachysylvia (canopy greenlets, Bonaparte 1850, type decurtata). Vireo remains unchanged except for the addition of the Tepui Vireo (nee Greenlet). The concatenated analysis placed the scrub greenlets (Hylophilus) sister to the peppershrikes (Cyclarhis), branching before the Shrike-Vireos (Vireolanius) which are basal to the remaining taxa.
It's unclear whether the two peppershrikes are reciprocally monophyletic. They are not in ND2, but are in the Z-linked genes studied by Slager et al. (2014). There is also a substantial amount of genetic distance within the Rufous-browed Peppershrike, Cyclarhis gujanensis, and more than one species might be involved. Further study is needed to clarify matters, especially as there is little vocal variation among the subspecies (Tubaro and Segura, 1994), with the main difference depending on climate and habitat (more high frequencies in closed habitats, and more bandwidth in the tropics).
Another potential split involves the Slaty-capped Shrike-Vireo, Vireolanius leucotis. The one sample from western Ecuador (subspecies mikettae, sampled from Lita, in Esmeraldas province) did not group with the birds east of the Andes in ND2.
The Golden Vireo (Golden Greenlet?) moves to Pachysylvia. The Red-fronted Greenlet, Tunchiornis rubrifrons, has been split from Tawny-crowned Greenlet, Tunchiornis ochraceiceps. Further research may support additional splits in the ochraceiceps complex, particularly between the Red-fronted Greenlets north and south of the Amazon.
When possible, the species tree includes available subgeneric names in italics for the various clades within Vireo. The four subgenera are spectacled vireos, Lanivireo (Baird 1858, type flavifrons); the eye-ringed vireos, Vireo (Vieillot 1808, type griseus); the eye-lined gilvus group, Melodivireo (Oberholser 1974, type gilvus); and the eye-lined olivaceus group, Vireosylva (Bonaparte, 1838, type olivaceus). And yes, that's the way Bonaparte spelled Vireosylva, so we're stuck with it.
Slager et al. (2014) found that the Central American Vireo, Vireo notius (inc. montanus), is basal in the Solitary Vireo group. It has accordingly been split from Plumbeous Vireo, Vireo plumbeus. Although genetic data is not available, the subspecies notius and montanus differ in appearance and are candidates for a further split.
Slager et al. (2014) found that Brown-capped Vireos of Mexico and northern Central America are genetically distinct from the remaining Brown-capped Vireos, Vireo leucophrys. Further research may lead to a split.
Warbling Vireo, Vireo gilvus, has been split into the monotypic Eastern Warbling-Vireo, Vireo gilvus, and Western Warbling-Vireo, Vireo swainsoni. They differ vocally, in morphometrics, molt and migration timing (Voelker and Rohwer, 1998) and the genetic distance supports their treatment as distinct species (Murray et al., 1994; Slager et al., 2014).
The treatment of the Red-eyed Vireo complex (flavoviridis through gracilirostris) follows Battey and Klicka et al. (2017) and uses both nuclear and mitochondrial DNA. I have split the South American Chivi Vireo, Vireo chivi, from Red-eyed Vireo, Vireo olivaceus. Slager et al. (2014) sampled Red-eyed Vireos from 6 locations and 18 Chivi Vireos and found that they are not sister taxa. Battey and Klicka (2017) concur. Interestingly, the Yucatan Vireo, Vireo magister, and the Black-whiskered Vireo Vireo altiloquus, the local breeding member of the Red-eyed complex here in Miami are better thought of thought of as northern forms of Chivi Vireo, rather than a southern forms of Red-eyed. The Yellow-green, Vireo flavoviridis, developed from the Red-eyed complex prior to the Red-eyed/Chivi split.
- Rufous-browed Peppershrike, Cyclarhis gujanensis
- Black-billed Peppershrike, Cyclarhis nigrirostris
- Gray-eyed Greenlet, Hylophilus amaurocephalus
- Rufous-crowned Greenlet, Hylophilus poicilotis
- Olivaceous Greenlet, Hylophilus olivaceus
- Ashy-headed Greenlet, Hylophilus pectoralis
- Scrub Greenlet, Hylophilus flavipes
- Gray-chested Greenlet, Hylophilus semicinereus
- Lemon-chested Greenlet, Hylophilus thoracicus
- Brown-headed Greenlet, Hylophilus brunneiceps
- Chestnut-sided Shrike-Vireo, Vireolanius melitophrys
- Green Shrike-Vireo, Vireolanius pulchellus
- Yellow-browed Shrike-Vireo, Vireolanius eximius
- Slaty-capped Shrike-Vireo, Vireolanius leucotis
- Tawny-crowned Greenlet, Tunchiornis ochraceiceps
- Red-fronted Greenlet, Tunchiornis rubrifrons
- Golden Vireo / Golden Greenlet, Pachysylvia hypochrysea
- Lesser Greenlet, Pachysylvia decurtata
- Dusky-capped Greenlet, Pachysylvia hypoxantha
- Buff-cheeked Greenlet, Pachysylvia muscicapina
- Golden-fronted Greenlet, Pachysylvia aurantiifrons
- Rufous-naped Greenlet, Pachysylvia semibrunnea
- Tepui Vireo, Vireo sclateri
- Philadelphia Vireo, Vireo philadelphicus
- Brown-capped Vireo, Vireo leucophrys
- Western Warbling-Vireo, Vireo swainsoni
- Eastern Warbling-Vireo, Vireo gilvus
- Yellow-green Vireo, Vireo flavoviridis
- Red-eyed Vireo, Vireo olivaceus
- Black-whiskered Vireo, Vireo altiloquus
- Yucatan Vireo, Vireo magister
- Chivi Vireo, Vireo chivi
- Noronha Vireo, Vireo gracilirostris
- Gray Vireo, Vireo vicinior
- Hutton's Vireo, Vireo huttoni
- Yellow-throated Vireo, Vireo flavifrons
- Yellow-winged Vireo, Vireo carmioli
- Choco Vireo, Vireo masteri
- Central American Vireo, Vireo notius
- Cassin's Vireo, Vireo cassinii
- Plumbeous Vireo, Vireo plumbeus
- Blue-headed Vireo, Vireo solitarius
- Blue Mountain Vireo, Vireo osburni
- Jamaican Vireo, Vireo modestus
- Flat-billed Vireo, Vireo nanus
- Bell's Vireo, Vireo bellii
- Puerto Rican Vireo, Vireo latimeri
- Slaty Vireo, Vireo brevipennis
- Black-capped Vireo, Vireo atricapilla
- Dwarf Vireo, Vireo nelsoni
- Mangrove Vireo, Vireo pallens
- San Andres Vireo, Vireo caribaeus
- Cozumel Vireo, Vireo bairdi
- White-eyed Vireo, Vireo griseus
- Thick-billed Vireo, Vireo crassirostris
- Cuban Vireo, Vireo gundlachii
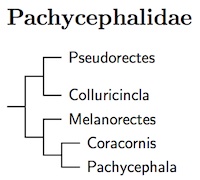
Pachycephalidae: Whistlers Swainson, 1831
5 genera, 61 species HBW-12
 |
| Click for Pachycephalidae species tree |
|---|
Genetic studies have done a number on the family. Although the core of it, the whistlers and shrike-thrushes, has remained unscathed, other taxa associated with the family have moved in or out. The pitohuis have been split into 4 genera, with Pitohui itself moving to Oriolidae and Ornorectes in Oreoicidae. The other three pitohuis have been retained in Pachycephalidae in two genera.
The shrike-thrushes have been included with the whistlers (Pachycephalidae) due to the analysis in Jønsson et al. (2008b, 2010a). They also found that the former Olive-flanked Whistler (Hylocitrea bonensis, now called Hylocitrea) is not only not a whistler, but not even in Corvida. It belongs somewhere in Passerida.
Rhagologus leucostigma, formerly the Mottled Whistler, has been moved to its own family under the name Mottled Berryhunter. See Aggerbeck et al. (2014), Jønsson et al. (2016), and Moyle et al. (2016).
Pitohuis: Although it turns out that they are not all that closely related, the pitohuis share an interesting characteristic. They're poisonous! (Dumbacher et al., 1992.) You can read more about these birds at Dumbacher's website and an article in American Birds. The exact relationships of these toxic species has not been exactly clear, so we shouldn't be entirely surprised that they have split apart. A paper by Jønsson et al. (2008a) found that the pitohuis are not that closely related to each other. Accordingly, they end up in four genera: Ornorectes (Oreoicidae), Pitohui (Oriolidae), Pseudorectes (Pachycephalidae), and Melanorectes (Pachycephalidae).
Shrike-thrushes: Based on Jønsson et al. (2008b, 2010a), The Sangihe Shrike-thrush has been transferred to Coracornis. Using different data sets, Dumbacher et al. (2008) and Jønsson et al. (2008b, 2010a) found that the Morningbird, which is sometimes placed in Colluricincla, sometimes in Pitohui, actually belongs in Pachycephala. The fact that the Morningbird is not in Colluricincla causes a change in the scientific name of the Sooty Shrike-thrush. With Colluricincla tenebrosa of Hartlaub and Finsch (1868) no longer using tenebrosa, the Sooty Shrike-thrush retakes the name tenebrosa (Rothschild 1911) which has priority over umbrina (Reichenow 1915).
Whistlers: The current arrangement of the whistlers is based on Jønsson et al. (2008c, 2009, 2010a, 2014) and Andersen et al. (2014b). I have not strictly followed the phylogeny in Anderseen et al., but have kept several lumped together several closely related taxa that may their phylogeny splits. Even so, I recognize 8 additional species:
- Bougainville Whistler, Pachycephala richardsi, split from Hooded Whistler, Pachycephala implicata.
- Louisiade Whistler, Pachycephala collaris, split from Bismarck Whistler, Pachycephala citreogaster.
- Rossell Whistler, Pachycephala rosseliana, split from Bismarck Whistler, Pachycephala citreogaster.
- Rennell Whistler, Pachycephala feminina, split from Oriole Whistler, Pachycephala orioloides.
- Temotu Whistler, Pachycephala vanikorensis, comprised of subspecies utupuae and ornata from the White-throated Whistler, Pachycephala vitiensis, and vanikorensis from the Melanesian Whistler, Pachycephala caledonica.
- Timor Whistler, Pachycephala calliope, split from Yellow-throated Whistler, Pachycephala macrorhyncha
- Wetar Whistler, Pachycephala arthuri, split from Yellow-throated Whistler, Pachycephala macrorhyncha. Note that arthuri is often synonymized with calliope.
- Babar Whistler, Pachycephala sharpei, split from Yellow-throated Whistler, Pachycephala macrorhyncha.
Further, the remainging (i.e. Fijian) races of the White-throated Whistler, Pachycephala vitiensis, are lumped into the Fiji Whistler, Pachycephala graeffii. The name vitiensis has priority, so the Fiji Whistler is now Pachycephala vitiensis.
Several whistler subspecies also move around. The Yellow-throated Whistler, Pachycephala macrorhyncha, loses dammeriana which joins the Mangrove Golden Whistler, Pachycephala melanura. Baliem Whistler, Pachycephala balim has been split from the Yellow-throated Whistler, Pachycephala macrorhyncha, where it had moved from Australian Golden Whistler, Pachycephala pectoralis (besides Andersen et al., 2014b, see also Beehler and Pratt, 2016). The Mangrove Golden Whistler, Pachycephala melanura loses dahli, which joins the Golden-backed Whistler, Pachycephala aurea, which becomes Pachycephala dahli due to priority.
Further, the Western Whistler, Pachycephala occidentalis, which consists of the Western Australian populations formerly known as fulignosa, has been split from the Australian Golden Whistler, Pachycephala pectoralis based on Joseph et al. (2014b). The eastern populations of fulignosa remain as a subspecies of pectoralis. See also Andersen et al. (2014b) and Jønsson et al. (2014).
Both the Melanesian Whistler, Pachycephala chlorura, and Oriole Whistler, Pachycephala orioloides may require further splitting in the future.
- White-bellied Pitohui, Pseudorectes incertus
- Rusty Pitohui, Pseudorectes ferrugineus
- Sooty Shrike-thrush, Colluricincla tenebrosa
- Little Shrike-thrush, Colluricincla megarhyncha
- Sandstone Shrike-thrush, Colluricincla woodwardi
- Bower's Shrike-thrush, Colluricincla boweri
- Gray Shrike-thrush, Colluricincla harmonica
- Black Pitohui, Melanorectes nigrescens
- Sangihe Shrike-thrush, Coracornis sanghirensis
- Maroon-backed Whistler, Coracornis raveni
- Bare-throated Whistler, Pachycephala nudigula
- Olive Whistler, Pachycephala olivacea
- Red-lored Whistler, Pachycephala rufogularis
- Gilbert's Whistler, Pachycephala inornata
- Regent Whistler, Pachycephala schlegelii
- Sclater's Whistler, Pachycephala soror
- New Caledonian Whistler, Pachycephala caledonica
- Bougainville Whistler, Pachycephala richardsi
- Hooded Whistler, Pachycephala implicata
- Louisiade Whistler, Pachycephala collaris
- Rossell Whistler, Pachycephala rosseliana
- Melanesian Whistler, Pachycephala chlorura
- Black-chinned Whistler, Pachycephala mentalis
- Yellow-throated Whistler, Pachycephala macrorhyncha
- Baliem Whistler, Pachycephala balim
- Western Whistler, Pachycephala occidentalis
- Australian Golden Whistler, Pachycephala pectoralis
- Golden-backed Whistler, Pachycephala dahli
- Mangrove Golden Whistler, Pachycephala melanura
- Bismarck Whistler, Pachycephala citreogaster
- Rennell Whistler, Pachycephala feminina
- Oriole Whistler, Pachycephala orioloides
- Temotu Whistler, Pachycephala vanikorensis
- Samoan Whistler, Pachycephala flavifrons
- Fiji Whistler, Pachycephala vitiensis
- Tongan Whistler, Pachycephala jacquinoti
- Morningbird, Pachycephala tenebrosa
- Vogelkop Whistler, Pachycephala meyeri
- Brown-backed Whistler, Pachycephala modesta
- Lorentz's Whistler, Pachycephala lorentzi
- Yellow-bellied Whistler, Pachycephala philippinensis
- Bornean Whistler, Pachycephala hypoxantha
- Sulphur-vented Whistler, Pachycephala sulfuriventer
- Mangrove Whistler, Pachycephala cinerea
- Green-backed Whistler, Pachycephala albiventris
- White-vented Whistler, Pachycephala homeyeri
- Timor Whistler, Pachycephala calliope
- Rusty-breasted Whistler, Pachycephala fulvotincta
- Wetar Whistler, Pachycephala arthuri
- Gray Whistler, Pachycephala simplex
- Island Whistler, Pachycephala phaionota
- Rusty Whistler, Pachycephala hyperythra
- Fawn-breasted Whistler, Pachycephala orpheus
- Babar Whistler, Pachycephala sharpei
- Wallacean Whistler, Pachycephala arctitorquis
- Drab Whistler, Pachycephala griseonota
- Cinnamon-breasted Whistler, Pachycephala johni
- Rufous Whistler, Pachycephala rufiventris
- Black-headed Whistler, Pachycephala monacha
- White-bellied Whistler, Pachycephala leucogastra
- White-breasted Whistler, Pachycephala lanioides
Oriolidae: Orioles, Figbirds Vigors, 1825
7 genera, 40 species HBW-13
 |
| Click for Oriolidae species tree |
|---|
The affinities of the recently extinct Piopios of New Zealand have long been uncertain. They have been variously considered birds-of-paradise, bowerbirds, thrushes, whistlers, and others. Sometimes they have been placed in their own family. There had been weak genetic evidence that they were close to bowerbirds. Two recent papers have now resolved the puzzle. Johansson et al. (2011) and Zuccon and Ericson (2012) found they are orioles!
The Oriolus orioles are widespread in the Old World, including Australasia. The other Oriolidae are confined to Australo-Papua (Pitohui, Sphecotheres) or New Zealand (Turnagra).
The arrangement here is primarily based on the analysis of Jønsson et al. (2010d), with some input from Johansson et al. (2011, 2016) and Zuccon and Ericson (2012). The genera are arranged as in Zuccon and Ericson (2012), which uses more genes, while Jønsson et al. (2010d) is followed for Oriolus. Jønsson et al. found evidence that Oriolus chinensis involves at least 3 species (there are 20 subspecies). It also is likely that steerii contains two or more species. The placement of crassirostris is a bit uncertain as it was not sampled.
Based on Dumbacher and Fleischer (2001), the Variable Pitohui has been split into three species: Southern Variable Pitohui, Pitohui uropygialis, Raja Ampat Pitohui, Pitohui cerviniventris, and Northern Variable Pitohui, Pitohui kirhocephalus. There's more information on pitohuis in the next section.
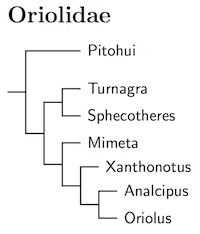
Based on Jønsson et al. (2016) as well as appearance, Oriolus has been separated into 4 genera. Brown Oriole though Tanimbar Oriole, which also have distinct skull morphology have been separated in genus Mimeta (Vigors and Horsfield 1827, type sagittata). Further, Dark-throated Oriole through Isabela Oriole have been placed in genus Xanthonotus (Bonaparte 1854, type xanthonotus). Finally, Black-and-crimson Oriole through Silver Oriole go in the genus Analcipus (Swainson 1831, type cruentus). The rest of the orioles remain in Oriolus (Linnaeus 1766, type oriolus)
The genetic results in Jønsson et al. (2010d) also support splitting Sunda Golden-Oriole, Oriolus maculatus, and Asian Golden-Oriole, Oriolus diffusus, from Black-naped Oriole, Oriolus chinensis. Jønsson et al. only included four of the twenty subspecies (melanisticus grouped with chinensis). Presumably Asian Golden-Oriole is monotypic, and mostly migratory. Sunda Golden-Oriole probably includes andamanensis, macrosurus, maculatus, mundus, richmondi, sipora lamprochryseus, and insularis, while Black-naped Oriole includes the Philippine races (chinensis, yamamurae, sulensis, and melanisticus). I'm not sure which group the Wallacean races belong to (sangirensis, formosus, celebensis, stresemanni, frontalis, bonratensis, and broderipi), and they are left in the Black-naped group until further information becomes available.
- Hooded Pitohui, Pitohui dichrous
- Southern Variable Pitohui, Pitohui uropygialis
- Raja Ampat Pitohui, Pitohui cerviniventris
- Northern Variable Pitohui, Pitohui kirhocephalus
- North Island Piopio, Turnagra tanagra
- South Island Piopio, Turnagra capensis
- Australasian Figbird, Sphecotheres vieilloti
- Green Figbird, Sphecotheres viridis
- Wetar Figbird, Sphecotheres hypoleucus
- Brown Oriole, Mimeta szalayi
- Olive-brown Oriole, Mimeta melanotis
- Green Oriole, Mimeta flavocinctus
- Olive-backed Oriole, Mimeta sagittata
- Dusky-brown Oriole, Mimeta phaeochroma
- Gray-collared Oriole, Mimeta forsteni
- Black-eared Oriole, Mimeta bouroensis
- Tanimbar Oriole, Mimeta decipiens
- Dark-throated Oriole, Xanthonotus xanthonotus
- Philippine Oriole, Xanthonotus steerii
- White-lored Oriole, Xanthonotus albiloris
- Isabela Oriole, Xanthonotus isabellae
- Black-and-crimson Oriole, Analcipus cruentus
- Black Oriole, Analcipus hosii
- Maroon Oriole, Analcipus traillii
- Silver Oriole, Analcipus mellianus
- Black-hooded Oriole, Oriolus xanthornus
- Green-headed Oriole, Oriolus chlorocephalus
- Western Oriole, Oriolus brachyrynchus
- Sao Tome Oriole, Oriolus crassirostris
- Ethiopian Oriole, Oriolus monacha
- Black-headed Oriole, Oriolus larvatus
- Black-winged Oriole, Oriolus nigripennis
- Mountain Oriole, Oriolus percivali
- African Golden-Oriole, Oriolus auratus
- Sunda Golden-Oriole, Oriolus maculatus
- Asian Golden-Oriole, Oriolus diffusus
- Black-naped Oriole, Oriolus chinensis
- Slender-billed Oriole, Oriolus tenuirostris
- Eurasian Golden-Oriole, Oriolus oriolus
- Indian Golden-Oriole, Oriolus kundoo