PALAEOGNATHAE Pycraft, 1900
The oldest major division among living birds is between the Palaeognathae and Neognathae. It was first recognized by Pycraft (1900). Kuhl et al. (2021) estimate that the split occurred around 94±4 mya. Stiller et al.'s (2024) figure ED-2 reads out at about 92.5±7.5 mya.
Although the ratites had long been considered related, the tinamous were thought to be distinct. Pycraft completed the group by adding the tinamous to the ratites, creating the Palaeognathae. This consists of the volant tinamous, the non-volant ratites (ostriches, rheas, emus, cassowaries, kiwis), and their extinct relatives, which include moas, elephant birds, and the Lithornithiformes (sometimes called false tinamous).
Traditionally, the Palaeognathae were divided into the flightless ratites and the volant tinamous. Some of the earlier generic evidence seemed to support this division (e.g., Haddrath and Baker, 2001).
This simple picture is now known to be wrong. An avalanche of more recent studies, including Chojnowski et al. (2008), Hackett et al. (2008), Harshman et al. (2008), Phillips et al. (2010), Faircloth et al. (2012), Haddrath and Baker (2012), J.V. Smith et al. (2013), Grealy et al. (2017), Yonezawa et al. (2017), Cloutier et al. (2019), Sackton et al. (2019), Urantówka et al. (2020), Simmons et al. (2022), and Takezaki (2023), have come to a very different conclusion. The ratites are not a natural grouping! The ratites are not monophyletic. This result is also supported by large-scale bird phylogenies such as Hackett et al. (2008), Prum et al. (2015), Kuhl et al. (2021), and Stiller et al. (2024).
Moas, Elephant Birds, and Lithornithiformes
The problem is that some of the ratites are more closely related to the volant tinamous than they are to other ratites. In particular, the ostriches are no more closely related to most of the other ratites than they are to the tinamous.
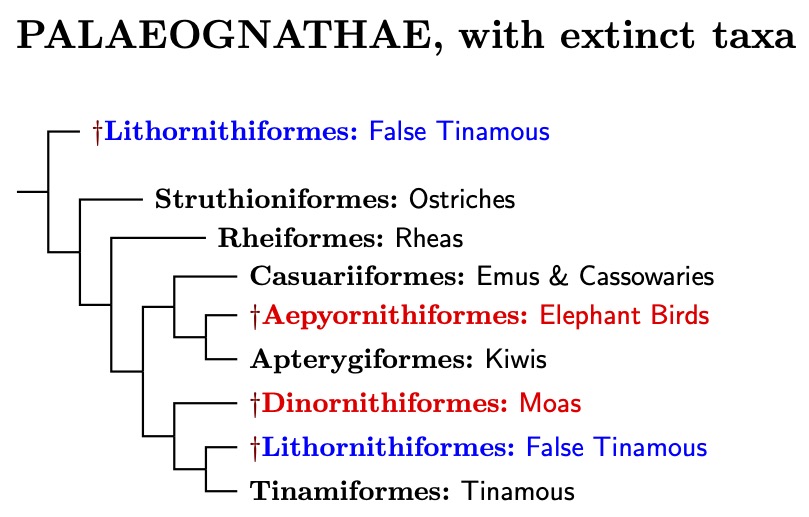 |
The diagram to the right shows the current situation. It includes not only living birds, but some extinct taxa. By using ancient DNA, we have been able to properly place the extinct Elephant Birds and Moas on the tree.
Unfortunately, ancient DNA only works if the fossils are not too ancient, so the extinct Lithornithiformes are a problem. Comparison of bones suggests that the most likely possibilities are that they are sister to the tinamous (Johnston, 2011; Nesbitt and Clarke, 2016), or are sister to all of the surviving paleognaths (Worthy et al., 2017; Yonezawa et al., 2017). I suspect the latter is correct. That is reflected in the accompanying diagram.
In fact, the situation with the Lithornithiformes may not be this simple. They may not even be a monophyletic group. See Nesbitt and Clarke (2016) and Widrig and Field (2022) for more on the Lithornithiformes.
What about the Rheas?
It's clear enough that the ostriches are the basal among the extant Palaeognathae. Once we get beyond the ostriches, things are not as clear-cut.
The remaining Palaeognathae comprise three groups: (1) rheas, (2) tinamous (and the recently extinct moas), and (3) cassowaries, emus, kiwis and the recently extinct elephant birds. These groups all separated over a short period of time, near the beginning of the Paleogene. E.g., Yonezawa et al. (2017) estimate the three groups separated from one another over a period of 4 million years.
So which group separated first? Even though it has been extensively studied, the short time period makes it hard to answer this question. Many analyses (e.g., Harshman et al., 2008; Phillips et al., 2010; J.V. Smith et al., 2013) favor putting the rheas first.
However, when Haddrath and Baker (2012) considered retroposons, the balance temporarily shifted to the tinamaou/moa clade, and that is the topology we formerly followed here. It is also the topology found by Cloutier et al. (2019), who used both coalescent and concatenated methods to address the question. The coalescent methods found that group (2), the tinamous and moas separated first. In contrast, concatenation favored group (1), the rheas.
Cloutier et al. attributed this discrepancy to incomplete lineage sorting. This is not surprising as the lineages all arose over a period of about 4 million years. Because coalescent methods are generally more accurate, I assumed the moas and tinamous separated first. TiF has followed this starting in December 2009 (version 2.52 of this file).
Somewhat more recent analyses such as Urantówka et al. (2020), Simmons et al. (2022), and Takezaki (2023) favor putting the rheas first. Simmons et al. (2022) considered three possibilities, which they color-coded:
- Green topology (1): Rheas sister to all Palaeognaths other than Ostriches.
- Red topology (2): Rheas sister to cassowaries, elephant birds, and kiwis.
- Blue topology (3): Rheas sister to moas and tinamous.
Figure 1 in Sackton et al. (2019) looks suspiciously like a hard polytomy. Mirarab et al. (2024) discovered that an apparent hard polyomy in Neoaves in the same time frame was due to a frozen segment of DNA. Stiller et al. (2024) developed a phylogeny that corrected for that. Lo and behold, it also resolves the Palaeognathae. The main tree in Stiller et al. supports the blue topology, where the Rheas are closer to the tinamous and moas. That is the topology now used by TiF. Questions still remain as the high guanine-cytosine content in the loci of the blue clades may have biased the result. In particular, the red topology cannot be completely ruled out.
Armed with a correct phylogeny (hopefully!), we can properly calibrate the divisions in the Palaeognathae. Stiller et al. (2024) did this, and found that all 5 Palaeognath orders dated to just before the end of the Cretaceous. I expect this really means just after, but it makes that point that all of the Palaeognath bird orders are truly ancient.
Flightlessness Common in Ratites
This topology suggests that flightlessness has evolved at least 6 times (!) in the Palaeognathae: in ostriches, rheas, moas, elephant birds, emus/cassowaries, and kiwis. The fact that the tinamous can fly implies flightlessness evolved at least four times. The distribution of the emus/cassowaries, elephant birds, kiwis suggests they each had volant ancestors, accounting for the other two times.
 |
There is further relevant DNA evidence indicating that the extinct elephant birds of Madagascar are the closest relatives of the kiwis (Mitchell et al., 2014b), with a common ancestor perhaps 50 mya. This is a problem for vicariance theories driven by continental drift because Madagascar likely separated from Gondwana considerably earlier, perhaps 120 mya. In that case, the ancestor of the elephant birds would have had to fly, and we can infer that flight was indpendently lost in the rheas, emu/cassorwary, elephant bird, and kiwi lineages, a total of 6 losses of flight. This is not an unreasonable number. One only has to consider all of the flightless rails to see that.
Paleognath Orders
The Palaeognathae are divided into several orders in recognition of the great antiquity of the various branches. Stiller et al. (2024) have all of the Palaeognath orders splitting shortly before the end of the Cretaceous period, although uncertainly does not rule out all splitting after the K/T (or K/Pg) boundary. Other sources come to different conclusions. Phillips et al. (2010) estimated the ostriches diverged from the rest of the Palaeognathae about 60-95 mya, while Chojnowski et al. (2008) dated this divergence around 64±22 mya, soon after the end of the Cretaceous. Haddrath and Baker (2012) put it at 73-119 mya. Mapping Jarvis et al. (2014) onto the phylogeny used here suggests that the split was about 84 mya (interval 58-96 mya).
The Cassowaries and Emus are much more closely related than the other paleognath groups, and are placed in a single family: Casuariidae. Although the Moas and Elephant Birds are not part of the main TiF list, a genus-level phylogeny of both is given in the tree diagram. The Moa tree is based on Bunce et al. (2009).
Based on calibrated trees, I suspect that only the ostriches and possibly false tinamous survived the Chicxulub meteorite that ended both the Cretaceous period and all of the non-avian dinosaurs.
STRUTHIONIFORMES Latham, 1790
Struthionidae: Ostriches Vigors, 1825
The authors for family-group names are mostly based on Bock (1994), while the authors for order-group names are primarily based on the series by Brodkorb (1963–1978). In both cases, additional sources have been consulted (e.g., Livezey and Zusi (2007)). In a number of cases, I have managed to examine the original (thank you Google Books!). The ICZN does not regulate order-group names. However, for parvorders (-ida), infraorders (-ides), suborders (-i), orders (-iformes), and superorders (-imorphae), I am attempting to follow similar rules. In particular, they are based on priority and the orginal use must been at an ordinal level and must be based on an included genus name (type genus) actually used by that author. The endings have been adjusted to modern usage.
1 genus, 2 species HBW-1
- Common Ostrich, Struthio camelus
- Somali Ostrich, Struthio molybdophanes
CASUARIIFORMES P.L. Sclater 1880
The Dromaiidae (Emus) have been merged into the Casuariidae (cassowaries) because the split between them seems fairly recent. The molecular dates in Phillips et al. (2010) and in Haddrath and Baker (2012) already suggested they were so closely related that they could be treated as a single family. More precise dating by Prum et al. (2015) placed the split at about 10 million years ago, too close to even rank them as subfamilies.
Casuariidae: Emus and Cassowaries Kaup, 1847
2 genera, 4 species HBW-1
Heupink et al. (2011) argue that the extinct King Island Emu, Dromaius ater, was quite closely related to the extinct Tasmanian subspecies of the Emu, and is best considered a dwarf subspecies of Dromaius novaehollandiae. The Kangaroo Island Emu, D. baudinianus, seems likely to have been no more different from the Emu than the King Island Emu was, so I am also considering it a subspecies of the Emu, D. novaehollandiae.
- Emu, Dromaius novaehollandiae
- Southern Cassowary, Casuarius casuarius
- Dwarf Cassowary, Casuarius bennetti
- Northern Cassowary, Casuarius unappendiculatus
APTERYGIFORMES Haeckel 1866

|
The order of the Kiwis reflects the phylogeny in Burbidge et al. (2003) and Shepherd et al. (2012). Burbidge et al. also provide evidence for recognizing 3 extant species of brown kiwi, as is done here. Shepherd et al. found evidence of an extinct northern clade of Little Spotted Kiwi that may deserve recognition as a separate species. The DNA samples of this potential species are from bones of unknown age.
Apterygidae: Kiwis G.R. Gray, 1840
1 genus, 5 species HBW-1
- Little Spotted Kiwi, Apteryx owenii
- Great Spotted Kiwi, Apteryx haastii
- Southern Brown Kiwi, Apteryx australis
- North Island Brown Kiwi, Apteryx mantelli
- Okarito Kiwi, Apteryx rowi
RHEIFORMES Forbes, 1884
Rheidae: Rheas Bonaparte, 1849
1 genus, 2 species HBW-1
- Greater Rhea, Rhea americana
- Lesser Rhea, Rhea pennata
TINAMIFORMES Huxley, 1872
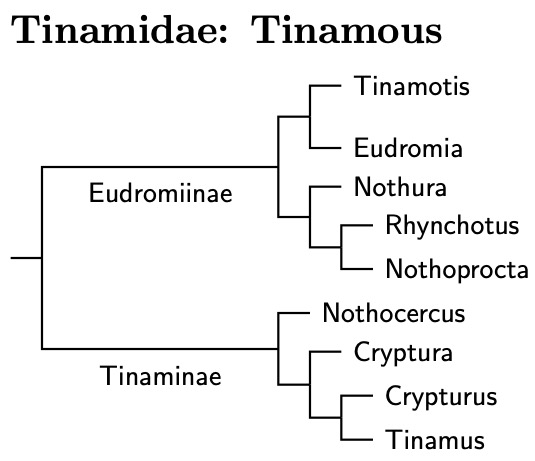
The Tinamiformes (and their extinct Moa cousins) form the remaining branch of the Palaeognathae. The taxonomy of the Tinamous is now based on Musher et al. (2024) and SACC.
Tinamidae: Tinamous G.R. Gray, 1840
10 genera, 47 species HBW-1
|
| Click for Tinamou species tree |
|---|
The age of the Tinamidae crown group, the descendents of the most recently common ancestor of the extant Tinamidae, is quite uncertain. On tree diagrams, this is the age of the split between the two subfamilies, Eudromiinae and Tinaminae, not when the Tinamade branch off from the other groups.
Stiller et al. (2024) put the age of the crown clade at around 47.5 million years ago. Almeida et al. give us two ages in Figure 3. The complete data (black lines in their Fig. 3) says 40 mya, but the RAG-2 gene (gray lines) says about 30 million years ago. To add to the confusion, Prum et al. (2015) puts it near 26 million years ago. We have some serious disagreement here.
When I look at the tinmous, both subfamilies seem pretty similar. I find it hard to believe they are divided by around 48 million years of evolution. Other groups that separated that long ago generally appear to be different. There is a bit more evidence we can use.
Stiller et al. (2024) used two calibration points. One put a minimum age of 16 mya on the Eudromiinae (estimated by Stiller et al. at 43.5 mya). The other put a minium age of 16 mya on the Tinamus-Cryptura clade (age est. at 42 mya). However, if we examine the supplementary material for Stiller et al., we find an anomaly. They give a plot of the estimated probability distribution for the age of Nothurinae, meaning Eudromiinae. It has a very tiny probability of being over 15 milion old. Even though the skewed t-distibution is fat-tailed, the probably of the age being over 47 million years is essentially zero.
It makes sense to think in terms of the gray lines on Figure 3 in Almeida et al. (2022) or even the somewhat earlier age estimates by Prum et al (2015). Prum et al. place the basal split in Eudromiinae at about 21 mya and the split between Cryptura and Tinamus at about 17 mya, which is even younger than the roughly 25 and 21 mya given by the gray lines. Suddenly the ages of the genera are looking much too.
If we think that the Prum et al. (2015) ages are close to correct, Tinamidaes naturally divides into two groups we can rank as subfamilies: Eudromiinae (Steppe Tinamous) and Tinaminae (Forest Tinamous). They may be a little older than is typical for subfamilies, but the similarities between suggest it is the most appropriate treatment.
The current arrangement of the Tinamidae follows Figure 2 of Musher et al. (2024). The current species tree marks the species with new DNA data using bright red asterisks. Only one species was not sampled, the Slaty-breasted Tinamou, Tinamus boucardi. Its placement reflects hybridization in Honduras with the Thicket Tinamou, Tinamus cinnamomeus (Monroe, 1968).
Besides reordering the tinamous, the Musher et al. tree suggests that the Andean Tinamou, Nothoprocta pentlandii, represents two non-sister species. In Birds of the High Andes, Fjeldså and Krabbe (1990) noted that the Andean Tinamou consists of a brownish group of subspecies and a grayish group. Accordingly, I've split the Andean Tinamou, Nothoprocta pentlandii, into two species:
- Brown Andean Tinamou, Nothoprocta oustaleti, containing subspecies fulvescens, oustaleti, niethammeri, and ambiguua and
- Gray Andean Tinamou, Nothoprocta pentlandii, containing the remaining subspecies: pentlandii, patriciae, doeringi, and mendozae.
Which Tinamus?
You may be puzzled by the way I use Tinamus. Wasn't Tinamus major the type? How can it be booted out of Tinamus? What happened?
The problem is that Tinamus was defined twice! The original definition was Hermann's in 1783. Seven years later Latham redefined it. Priority goes to Hermann, but Latham's version is the one usually used. Sometimes Hermann's name is even attached to Latham's Tinamus and major is claimed as the type, by subsequent designation.
But this is wrong! The rule is that the type must be among the species named in the original description. The only name Hermann mentioned was soui. Although he also referred to a description of tinamous in Buffon, who included major and a few others, he did not explicitly mention the species included in Buffon.
Names included only by reference cannot be among the originally included nominal species. The ICZN rules are quite clear on this (Art. 67.2.3). They are also clear that the type must be an originally included nominal species. That leaves soui as the only possible type species for Tinamus Hermann. No subsequent designation is possible.
I don't know the complete story, but the genus Tinamus had long been attributed to Latham (1790), with type major. Eventually, Hermann's 1783 work, Tabula affinitatum animalium, was noticed. Hermann was given priority. Apstein's 1915 fixation of the type as major for Tinamus Hermann was widely adopted, even by H↦M-4. However, that doesn't really solve the problem as major was not an originally included species. Only soui qualifies. In other words Apstein's solution doesn't work.
This problem with Apstein's fix was pointed out in BirdForum by Laurent Raty. Some of the details are also mentioned on the Richmond cards for Tinamus here and here, where the reference to Buffon is accepted. And yes, Buffon is the same Buffon as in Buffon's needle problem. Using the (now incorrect) inclusion of Buffon's species, Apstein attempted to fix the type as major in 1915. I'm not sure when the code explicitly barred inclusion by reference, so that might have been ok in 1915. It's not ok now.
No one seemed willing to follow the ICZN Code here, so I thought I would stir the pot by doing so. One problem with the Code is that it can sometimes cause (scientific) naming chaos. Ideally, the ICZN would decide in favor of stability here and elsewhere (e.g., Sulidae) and preserve traditional usage. I notice that Bertelli et al. (2025) has now also made the change to Tinamus Hermann.
Cryptura and Crypturus
As major is also the type of Cryptura, I originally put the Tinamus Latham species in Cryptura. Most of what had been called Crypturellus became the new Tinamus (i.e., Hermann's), with the rest becoming genus Crypturus (cinereus, berlepschi, ptaritepui). Yes, it's confusing, but it's long past time to correct this.
Unfortunately, my previous attempt also suffers a problem. I had assumed that cinereus was the type of Crypturus, based on Salvadori (1895). However, this was incorrect. Illiger, who defined Crypturus, felt that names without a Latin or Greek root should be rejected. That means we must read Illiger (1811) as defining a replacement name for Tinamus: Crypturus. The type of Tinamus was later made Tinamus brasiliensis Latham = Tetrao major Gmelin by Gray. This means it must also be the type of Crypturus.
I'd previously used Cryptura Vieillot 1816 for the major group. That has now been replaced by Crypturus Illiger. With Crypturus no longer available for the cinereus group, we turn to Crypturornis Oberholser 1922 for it.
Finally, Bertelli, Almeida, and Giannini (2025) proposed the name Paranothoprocta for cinerascens. This makes sense as it both looks quite different from the two Rhynchotus tinamous, and the most recent common ancestor may have lived over 15 may, this makes a lot of sense.
Tabel of Tinamidae Type Species
The table below lists the type species for each of the genera used by the current TiF list. Type species are noted on the species tree by leading black 5-pointed stars.
| Genus | Type Species | Author | Year | |
|---|---|---|---|---|
| Tinamotis | pentlandii | Vigors | 1837 | |
| Eudromia | elegans | I. Geoffroy Saint-Hilaire | 1832 | |
| Nothura | boraquira | Wagler | 1827 | |
| Paranothoprocta | cinerascens | Bertelli, Almeida, & Giannini | 2025 | |
| Rhynchotus | rufescens | von Spix | 1825 | |
| Nothoprocta | perdicaria | Sclater & Salvin | 1873 | |
| Nothocercus | julius | Bonaparte | 1856 | |
| Crypturus | major | Illiger | 1811 | |
| Crypturornis | cinereus | Oberholser | 1922 | |
| Tinamus | soui | Hermann | 1783 | |
In sum, I've made the following genus changes compared to the earlier TiF version of the Tinamous (versions 3.03, March 18, 2018 and earlier) which had the same genera as version 13.2 of the IOC list:
- Taoniscus has been absorbed into Nothura,
- Tinamus Latham 1790 is renamed Crypturus,
- Paranothoprocta has been split from Rhynchotus
- Berlepsch's Tinamou, Crypturellus berlepschi, Cinereous Tinamou, Crypturellus cinereus, and Tepui Tinamou, Crypturellus ptaritepui, have been moved to Crypturornis
- The remainder of Crypturellus is now called Tinamus Hermann 1783.
What about the Chaco Nothura?
Hayes et al. (2018) showed that there is no reason to consider the Chaco Nothura, Nothura chacoensis, distinct from the Spotted Nothura, Nothura maculosa. As a result, I now consider the Chaco Nothura to be a subspecies of the Spotted Nothura, Nothura maculosa.
Tinamidae species list
Eudromiinae: Steppe Tinamous Bonaparte 1854
- Puna Tinamou, Tinamotis pentlandii
- Patagonian Tinamou, Tinamotis ingoufi
- Quebracho Crested-Tinamou, Eudromia formosa
- Elegant Crested-Tinamou, Eudromia elegans
- White-bellied Nothura, Nothura boraquira
- Dwarf Tinamou, Nothura nanus
- Lesser Nothura, Nothura minor
- Darwin's Nothura, Nothura darwinii
- Spotted Nothura, Nothura maculosa
- Brushland Tinamou, Paranothoprocta cinerascens
- Red-winged Tinamou, Rhynchotus rufescens
- Huayco Tinamou, Rhynchotus maculicollis
- Curve-billed Tinamou, Nothoprocta curvirostris
- Ornate Tinamou, Nothoprocta ornata
- Brown Andean Tinamou, Nothoprocta oustaleti
- Taczanowski's Tinamou, Nothoprocta taczanowskii
- Gray Andean Tinamou, Nothoprocta pentlandii
- Chilean Tinamou, Nothoprocta perdicaria
Tinaminae: Forest Tinamous G.R. Gray, 1840
- Tawny-breasted Tinamou, Nothocercus julius
- Highland Tinamou, Nothocercus bonapartei
- Hooded Tinamou, Nothocercus nigrocapillus
- Great Tinamou, Crypturus major
- Gray Tinamou, Crypturus tao
- Solitary Tinamou, Crypturus solitarius
- White-throated Tinamou, Crypturus guttatus
- Black Tinamou, Crypturus osgoodi
- Berlepsch's Tinamou, Crypturornis berlepschi
- Cinereous Tinamou, Crypturornis cinereus
- Tepui Tinamou, Crypturornis ptaritepui
- Brown Tinamou, Tinamus obsoletus
- Tataupa Tinamou, Tinamus tataupa
- Small-billed Tinamou, Tinamus parvirostris
- Variegated Tinamou, Tinamus variegatus
- Little Tinamou, Tinamus soui
- Bartlett's Tinamou, Tinamus bartletti
- Barred Tinamou, Tinamus casiquiare
- Rusty Tinamou, Tinamus brevirostris
- Undulated Tinamou, Tinamus undulatus
- Yellow-legged Tinamou, Tinamus noctivagus
- Black-capped Tinamou, Tinamus atrocapillus
- Pale-browed Tinamou, Tinamus transfasciatus
- Thicket Tinamou, Tinamus cinnamomeus
- Slaty-breasted Tinamou, Tinamus boucardi
- Choco Tinamou, Tinamus kerriae
- Red-legged Tinamou, Tinamus erythropus
- Gray-legged Tinamou, Tinamus duidae
- Brazilian Tinamou, Tinamus strigulosus