CHARADRIIFORMES Huxley, 1867
The order Charadriiformes includes 22 families. These are divided into over 120 genera and roughly 400 species. The birds included range from shorebirds to alcids, terns and gulls. The Charadriiformes have been carefully studied in recent years and DNA methods have proven especially effective at unraveling the taxonomy. We not only know how most of the various families relate, but we also have a good handle on many of the genera. There are a few exceptions, including the lapwings where too many taxa remain unsampled.
There is a lot of recent evidence for monophyly of the Charadriiformes as constituted here. This includes Ericson et al. (2003a), Paton et al. (2003), Cracraft et al. (2004), Thomas et al. (2004a), Paton and Baker (2006), Baker et al. (2007), Fain and Houde (2007), Kuhl et al. (2021), Černý, and Natale (2022), and Stiller et al. (2024).
Many studies have found that gulls and alcids are closely related to the shorebirds. Collectively, these analyses have made it quite clear that the sandgrouse (Pteroclidae) and bustards (Otididae) are not Charadriiformes. They have also shown that the various buttonquail (Turnicidae) and the plains-wanderer (Pedionomidae) are Charadriiformes. The position of the buttonquail family is also supported by morphology (Mayr, 2008a).
Historically, the buttonquails (Turnicidae) were the last family to join the Charadriiformes. At one point, they were thought to be related to quail, and were placed in the Galliformes (e.g., Gadow, 1892). However, they are actually quite different from true quail and were later grouped with the cranes and rails (Gruiformes). Sibley, Ahlquist, and Monroe (1988) listed them in their own separate group. Van Tuinen, Sibley, and Hedges (2000) had Turnix sister to Larus, but not Charadriius, which was also nearby. Paton et al. (2003), in their study of Charadriiformes, attempted to use Turnix as an outgroup. Imagine their surprise when they found their outgroup was nested within Charadriiformes! Indeed, they found it was sister to a group containing the gulls, as it is today. By now, there are no plausible additions to or deletions from the Charadriiformes. They have all be tested genetically, and adding the Turnicidae completed the process.
Stiller et al. (2024) estimated that the most recent common ancestor of the Charadriiformes and their sister group, the Gruiformes, lived about 64.5 million years ago (mya).
Temporal Assignment of Linnaean Ranks
Sibley and Monroe's checklist (Sibley and Monroe, 1990; Monroe and Sibley, 1993) tried to make the use of Linnaean ranks consistent throughout the class Aves by using a measure of genetic distance to define the ranks. This standard meant that many passerine families had to be defined extremely broadly, with the traditional families relegated to subfamily or even tribal status. By modern counts, their Fringillidae would contain well over a thousand species. This proved unworkable. Saying a bird is in Fringillidae was just too uninformative, and has not been followed by contemporary ornithologists.
I think the idea of using genetic distance, or better, estimates of group age to insure consistency of the ranks is a good one. However, I think Sibley and Monroe's big mistake was to attempt to apply the ranks uniformly over time. Rather, I think it is also useful to take into account how speciose our various groups are, and that this can be accomplished by setting up (fuzzy) age bands for each rank. These age bands can vary depending on where were are in the avian tree. The age bands will be newer for highly speciose groups so that families end up being more reasonably sized. I think this will work well for Neornithes. Applying Linnaean ranks to fossils involves too much complication. Paleontologists have developed an unranked system called PhyloCode to deal with that. Some of the higher-order groups on the TiF list are PhyloCode names.
Linnaean Ranks for Shorebirds
The Charadriiformes consist of 22 families, by far the most for any order other than Passeriformes (144). To help make the taxonomy clear, I've used three Linnaean-style ranks between orders and families. I also use two additional ranks between family and genus on some of the family pages.
The names below are all based on the genus Charadrius, to illustrate how the names of the ranks are formed. The ending ‑us is replaced by the appropriate rank ending.
| Rank | Name | Suffix |
|---|---|---|
| Order | Charadriiformes | ‑-iformes |
| Suborder | Chardrii | ‑i |
| Parvorder | Charadriida | ‑ida |
| Superfamily | Charadrioidea | ‑oidea |
| Family | Charadriidae | ‑idae |
| Subfamily | Charadriinae | ‑inae, and |
| Tribe | Charadiini | ‑ini. |
Additional ranks will be used in the Passeriformes, include infraorder (suffix ‑ides) and the rarely used epifamily (suffix ‑oidae).
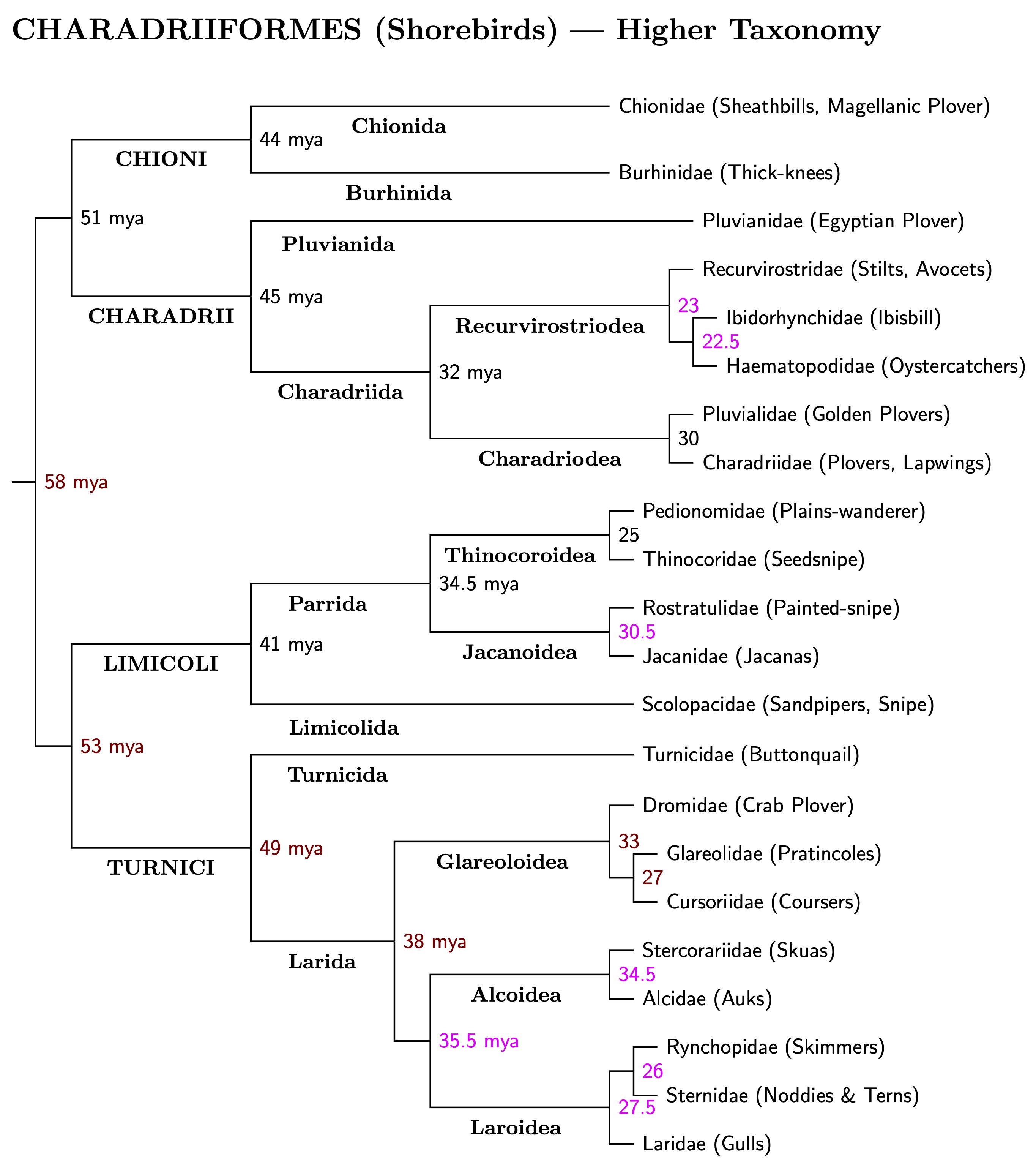
Stiller et al. (2024) estimate the Charadriiformes originated at the beginning of the Cenozoic, or more specifically, the beginning of the Palaeocene. Only one shorebird lineage seems to have survived from then. About 58 mya it split into two surviving lineages. Each of them split again around 51-53 mya. Our first temporal band applies to suborders of the Charadriiformes, and I place it in the early Eocene, ranging from 50 to 56 mya.
Accordingly, I've divided the Charadriiformes into four suborders: Chioni, Charadrii, Limicoli, and Turnici. The tree below shows how it all fits together, including the division into suborders (ending with ‑i), infraorders (ending with ‑ides), parvorders (‑ida), and superfamilies (‑oidea), and finally the 22 shorebird families (‑idae).
Notice that not every division of the shorebird tree gets a name. The basal split does not. Neither does the group containing Alcoidea and Laroidea, but not Glareoida.
The next temporal band, for parvorders, ranges from 40 to 50 mya. The third temporal band applies to superfamilies, and runs from 30 to 40 mya. It includes the later Eocene and the early Oligocene. Finally, although the branches may be older, the family band is 20 and 30 mya. This means the most recent common ancestors of each shorebird family lived between 20 and 30 mya.
You may wonder how I came up with these age ranges. I started with the families. I had noticed some time ago that a number of bird families (except the nine-primaried oscines) often seemed to have ages of around 20 million years or so, and that non-passerine families tended to be a bit older. What's different now is that I'm imposing it as a rule. The Charadriiformes are its first test. The bands for the rest made sense once I'd decided on the family age band.
Calibrated Ages of Taxa
A problem that has cropped up in large-scale studies of birds is the fact that standard molecular clocks have performed poorly. One way to try of overcome this problem is to use fossil calibration. One early calibration effort was van Tuinen and Hedges (2001). As is usual with calibration studies, their ages were too old rather than too young (see their Fig. 3). This has been repeatedly seen in calibration efforts, including that of Baker et al. (2007), who focused on the Charadriiformes.
The basic idea of calibration is to take a genetic tree and then think of the different species in terms of skeletal characters rather than DNA, and find the characters that are salient. Hopefully, we can then place fossils on the DNA tree. That allows us to find minimum ages for the splits in the DNA trees. One possible complication is that multiple DNA sequences can generate the same skeletal characteristics (convergent evolution). This can result in misplaced fossil taxa. If the taxa are not misplaced, we get a time scale for the various splits in the tree.
The fossil from Černý and Natale's calibration point 14 may be an example of this. The fossil is 55.88 million years old. Attempts to place it on a phylogentic tree using skeletal characters puts in in the Chionida. This is highly unlikely given that it seems way too old for the Chionida. Their apparent age is 44 mya. So something is wrong, and I strongly suspect there's a false signal from the skeletal characteristics (this is a reasonably common problem). I note that Černý and Natale treat it as only as a Charadriiforme, not a member of Chionida.
Although calibration methods have been improved, there's still considerable uncertainty concerning the age of the shorebird clades and species. Moreover, in practice, calibration seems biased toward older ages. This can be overcome if you have enough good calibration fossils. Generally, we don't. For example, Černý and Natale (2022) use 14 fossils to calibrate their shorebird tree. This is more than many studies use. More importantly, they made a real effort to use properly vetted calibration fossils.
Vetting is especially important when dealing older paleontological literature, but even some relatively recent papers have issues when trying to classify fossil birds (e.g., Hope, 2002). The solution involves analyzing them phylogenetically, using features of bones rather than DNA to place them on a phylogenetic tree of living birds constructed using DNA. This type of phylogenetic method is not perfect, but it wasn't long ago that paleontologists often had strange ideas about the relation between avian lineage fossils and modern bird taxa.
Kudos to Černý and Natale for being careful to calibrate using fossils that could be accurately placed on the avian tree. The DNA did not get a similar level of scrutiny in their unpublished draft, but they corrected that in the published version (yes, sometimes DNA is not what you think it is).
I think even Černý and Natale (2022) still have many dates that are too old. Many of these occur in branches far from calibration points. Where there are nearby calibration points, the dating is usually considerably improved compared with other calibrated studies of the Charadriiformes. Compare Paton et al. (2003) or Baker et al. (2007). One clue that there are still some problems involves the prolonged lack of speciation in much of the Černý and Natale tree (look at the tips). This is an artifact that often occurs when calibrating: time is too regularly spaced out along branches of the phylogenetic tree.
Černý and Natale's (2022) use of more calibration points has had an beneficial effect and some parts of the tree look pretty reasonable. Others, including the Charadriidae, don't. It shows the symptoms mentioned above. The branch splits look too regular, and theer's little recent branching.
In some cases I'm using Černý and Natale's age estimates rather than Stiller et al. (2024) when designating higher-level taxa, and also when deciding whether to use additional genera (I generally consider shorebird families over-lumped at the genus level). For dates at the family level and above, I now rely more on the comprehensive analysis of Stiller et al. with some assistance from Kuhl et al. (2021) and Prum et al. (2015).
Calibrating Alcoidea and Laroidea
Černý and Natale (2022) used 6 of their 14 calibration points for the alcids, making it the best-calibrated part of the shorebird tree. However, there is a problem. Their calibration doesn't match up well with Stiller et al. (2024). After digging a bit, I decided Černý and Natale were right. They've shown that the dates in Stiller et al. are wrong, at least for alcids and related birds (the suborder Turnici).
So how can be so sure Stiller et al. are wrong? The oldest alcid fossils are older than the entire Aclidae are in Stiller et al! Stiller et al. concluded that the Alcidae were about 25 million years old. However, Černý and Natale's calibration point 6 is a fossil alcid that at least 34.44 million years old. That's nearly 10 million older than alcids are supposed to be. Oops!
So we have to recalibrate. I've ended up adopting Černý and Natale's dates for the Alcoidea and its sister group Laroidea (the magenta dates) and adjusting the dates upstream of them upward (the dark red dates). This is necessary because adding calibration point 6 will change the estimates of date on its branch of the tree, broadly construed. I'm taking that to mean the Turnici. There were also some smaller discrepancies involving the superfamily Haematopodoidea and the painted-snipe (Rostratulidae).
Higher Taxonomy of Shorebirds
The figure below gives an overview at family-level and above. The ages in black are from Stiller et al. (2024). The ages in magenta (pink) came from Černý and Natale (2022), while those in red represent a blend from both sources. Notice how the higher ranks are in the appropriate bands.
Clicking on higher taxonomy diagram leads to a 3-page genus-level Charadriiformes tree, which goes into more detail. It is primarily based on Černý and Natale (2022). Besides the suborders, parvorders, superfamilies, and families included in the higher taxonomy tree, the detailed tree also includes subfamilies (‑inae), and genera. Details on species are found on the individual family trees included in the family accounts. One of the family trees also divides subfamilies into tribes.
 |
| Click for Charadriiformes genus level phylogeny |
|---|
Superfamilies that would contain only one family are not marked on the tree. One quirk of naming ordinal and higher level taxa is that ICZN rules don't apply. My use of Limicoli rather than Scolopaci is intended to highlight the problem.
Černý and Natale's genetic phylogenies: The basic structure of the shorebird order is now pretty well worked out. The genetic phylogenies in Černý and Natale (2022) have done much to clarify the details, often down to species level. The total evidence phylogeny from their paper has been used only sparingly as it is hard to tell when non-genetic evidence is useful, and when it just adds noise. Their two genetic phylogenies can be found in the supplemental material, Figures A.31-A.32 and A.33-A.34.
I've been using Černý and Natale's phylogenies since their preprint appeared. The phylogenies have improved in the published version and now seem pretty solid. However, the large white-headed Larus gulls are an exception. This should be no surprise to any birder that closely studies gulls. I think the problem is that recent speciation has led to incomplete lineage sorting driven by frequent, and often ongoing, hybridization. Olympic and Nelson's Gulls come to mind, but there are many others. See the Wikipedia page on hybrid gulls. Sorting this out probably requires additional data and perhaps new methods of analysis.
Chioni Sharpe, 1890
The suborder Chioni contains two distantly related familes: Burhinidae (thick-knees and stone-curlews) and Chionidae (sheathbills and Magellanic plover). The split between the Chionidae and Burhinidae families is dates to 44 mya, according to Stiller et al. (2024) The shorebird suborder Charadrii contains the closest relatives of the Chioni, even though Stiller et al. estimate they've been separated for about 51 million years.
We could list trivial parvorders Chionida and Burhinida, and equally trivial superfamilies Chionoidea and Burhinoidea. As there's no gain from doing so, we don't. We skip directly to the two families.
Chionidae: Sheathbills Lesson, 1828
2 genera, 3 species HBW-3
It has become increasingly clear that the Magellanic Plover is fairly closely related to the Sheathbills. Černý and Natale (2022) estimated their common ancestor lived around 16 million years ago, and Stiller et al. estimated it lived a bit more recently, at about 13 mya. With such a recent common ancestor, these birds don't belong in separate families. I've put them in subfamilies because sheathbills and the Magellanic Plover are so different. The combined family is called Chionidae because it has priority over Pluvianellidae.
Pluvianellinae: Magellanic Plover Jehl, 1975
- Magellanic Plover, Pluvianellus socialis
Chioninae: Sheathbills Lesson, 1828
- Snowy Sheathbill, Chionis albus
- Black-faced Sheathbill, Chionis minor
Burhinidae: Thick-knees Mathews, 1912 (1840)
4 genera, 10 species HBW-3
Černý and Natale's preprint (2021) caused me a problem when it came to the thick-knees. They corrected it in the published version Černý and Natale (2022). But as it involves a continuing issue for others, I've decided to retain a discussion of the issue.
The issue can summed up in two questions: What is Burhinus magnirostris? And what is Esacus magnirostris. The confusion started when Burhinus magnirostris was variously applied to both the Beach Stone-Curlew and the Bush Stone-Curlew. So how did two birds get the same scientific name? Which bird did the earlier version of Černý and Natale refer to?
Two Birds called Burhinus magnirostris
Although the Beach Stone-Curlew, often called Esacus magnirostris, and the Bush Stone-Curlew, sometimes called Burhinus magnirostris, are two entirely different birds, they are both magnirostris. Here are the original forms of these names:
- Beach Stone-Curlew: Oedicnemus magnirostris Vieillot 1818
- Bush Stone-Curlew: Charadrius magnirostris Latham 1801
So what's going on here? And why is the Bush Stone-Curlew now often called Burhinus grallarius, as in Černý and Natale (2022)? It all has to do with the rules governing priority of names, now controlled by the ICZN Code, overseen by the International Commission on Zoological Nomenclature.
We'll start with the easy one first. When the Bush Stone-Curlew was originally named by Latham in 1801 he gave it three names, the other two being grallarius and frenatus. Since all three were used in the same publication, none was older. All are equally the oldest name. In a case like this, someone has to pick the name, act as a first reviser. Well, evidently no one did, at least explicitly. No one pointed to the three names and said “this one”!
So magnirostris came into use for the Bush Stone-Curlew. Finally, Gould (1845) picked grallarius. This took a long time to come into regular use, but has been followed by much of the recent literature. Nonetheless, one sometimes still sees Burhinus magnirostris for the Bush Stone-Curlew.
Since it is in a different genus, one might think that this would allow us to use magnirostris for the Beach Stone-Curlew. However, both are sometimes placed in the genus Burhinus. In that case, Latham's name still has priority over Vieillot's even though it is not in use. That means that Burhinus magnirostris cannot be used for the Beach Stone-Curlew, although it sometimes is, even in the 21st century.
Technically, Vieillot's name is a secondary junior homonym of Latham's. It's junior because Latham got there first, and secondary because Latham's name is not actually in use (officially). Nonetheless, that is enough to prevent that name from being legitimately used when both are in the same genus.
Under the current rules, that would mean that magnirostris would be available for the Beach Stone-Curlew provided that it was not in the same genus as the Bush Stone-Curlew. So the Beach Stone-Curlew could be called Esacus magnirostris. The priority of Burhinus over Esacus would mean that Bush Stone-Curlew could not be placed in genus Esacus.
However, the rule used to be different. In the interest of maintaining the same name when possible, decisions made under the previous rules are still binding. The old rule held prior to 1961, and would have prohibited use of magnirostris for the Beach Stone-Curlew under any circumstances. This holds provided someone published a paper that pointed it out. Just as someone (eventually Gould) had to choose the right name for the Bush Stone-Curlew, someone needed to address this problem in a published work.
That someone was Meinertzhagen. In 1924, well before the cutoff of 1961. she put all of the Burhinidae in a single genus, Burhinus. Although almost eighty years had passed since Gould (1845), magnirostris was still being used for both species and Meinertzhagen decided to do something about it.
In her species account for the Beach Stone-Curlew, Meinertzhagen rejected Wagler's name giganteus as unidentifiable (pg. 351). She also rejected the name major. Next in line was neglectus Mathews, 1912. Meinertzhagen was able to examine the type specimen. She verified its identity, and the Beach Stone-Curlew became Burhinus neglectus! Since this happened before 1961, Article 59.3 of the Code applies. This makes the application of magnirostris to the Beach Stone-Curlew permanently invalid. Unfortunately, it doesn't stop people from using it, which can contribute to the confusion.
An internet search reveals that the name neglectus has gotten some use, but the Beach Stone-Curlew is often placed in Esacus and called Esacus magnirostris, contrary to Article 59.3. One example is Hayman et al. (1986), which uses magnirostris for both Bush and Beach Stone-Curlews. Another is the IOC, as of mid-August 2024.
Relatively recent discussions of this issue can be found in Christidis and Boles (1994, 2008) and Hume's (1996) article on Burhinidae in HBW-3 (pg. 350) as well as a thread on BirdForum. That's why you see Beach Stone-Curlew named Esacus neglectus in the list below. Hope I have it all straight!
That brings us back to Černý and Natale (2021).
What did Černý and Natale mean by Burhinus magnirostris?
So what did Černý and Natale (2021) mean by Burhinus magnirostris? Like Meinertzhagen (1924), they included all of the thick-knees in Burhinus. We start with Figure A.2 of the supplementary material. This shows they do not use any genetic information from it. So the only information is morphological, from Strauch (1978). According to a BirdForum post by Černý, it is the Beach Stone-Curlew, called Esacus magnirostris by Strauch that they mean by Burhinus magnirostris. He noted they also used morphological data for the Bush Stone-Curlew, called Burhinus magnirostris by Strauch, which we call Burhinus grallarius, and that they were careful to keep them straight. That makes it clear that Černý and Natale intended that Burhinus magnirostris refer to the Beach Stone-Curlew and that Burhinus grallarius refer to the Bush Stone-Curlew. You'd think that would resolve the problem.
Life is not so simple. The problem is not fully resolved because Černý and Natale also used sequences from GenBank from what GenBank listed as Bush Thick-knee, Burhinus grallarius (aka Bush Stone-Curlew). Looking in GenBank, I found a number of genes listed as Burhinus grallarius, the Bush Thick-knee. These genes were sequenced variously for the papers by Paton et al. (2003), Paton and Baker (2006), and Baker et al. (2012). They ultimately sampled three specimens from the Royal Ontario Museum in Toronto. But the papers don't mention obtaining any genes from specimens of the Bush Thick-knee. The papers tell us the birds involved were Burhinus magnirostris! They clarify its identity by giving the English name, Beach Thick-knee (i.e., Beach Stone-Curlew). Oops!
All of the DNA they extracted refers to vouchered specimens AJB6174, AJB6175, and AJB6177 (AJB = Allan J. Baker). In fact, the only real Bush Thick-knee genes represented in GenBank seem to be microsatellite data. There's one other submission that claims to be cyt-b from a Bush Thick-knee. However, that DNA sample is from blood taken from a mosquito that bit a bird. The bird was identified by noticing the partial cyt-b sample was identical to a portion of the cyt-b sample labelled voucher AJB6177, and sampled by Paton and Baker (2006). In other words, it's really a Beach Stone-Curlew too.
So the answer is that in their preprint (Černý and Natale, 2021), Burhinus magnirostris referred to the morphological Beach Stone-Curlew, while Burhinus grallarius referred to a chimera consisting of DNA from the Beach Stone-Curlew, with the morphology of a Bush Stone-Curlew. All these difficulties stem from using the same name for two birds. Fortunately, the published version, Černý and Natale (2022) does not suffer from this confusion.
The DNA barcoding database BOLD seems to include both Beach and Bush Stone-Curlews. See Laurent Raty's discussion on BirdForum.
Buhinidae Taxonomic Notes
Thick-knees: Based on Paton et al. (2003) and Černý and Natale (2022), there seem to be four main clades in Burhinus. I treat each as a separate genus.
- The New World clade includes the Double-striped Thick-knee, Hesperoburhinus bistriatus, and the Peruvian Thick-knee, Hesperoburhinus superciliaris. Hesperoburhinus (Černý, van Els, Natale, & Gregory, 2023), has type bistriatus.
- The Bush Stone-Curlew, Burhinus grallarius. This is Burhinus, now reduced to a single species.
- The Great and Beach Stone-Curlews are still in genus Esacus.
- The remaining five species, formerly included in Burhinus, take the name Oedicnemus (Temminck 1815, type oedicnemus).
 |
| Click for Burhinidae species tree |
|---|
The relation between the other three clades is still unresolved because the Bush Stone-Curlew remains unsampled. It is believed to be close to Esacus, so I treat the three clades as a trichomoty. I restrict Burhinus to a single species, the Bush Stone-Curlew, Burhinus grallarius. A second clade is Esacus, containing the Beach and Great Stone-Curlews. Finally, the third clade contains the other species. It takes the genus name Oedicnemus (Temminck 1815, type oedicnemus).
Although Černý and Natale (2022) had no DNA from either Esacus, Paton et al. (2003) sequenced the RAG-1 gene for the Beach Stone-Curlew, Esacus magnirostris (i.e., neglectus), and several other thick-knees. The sample (AY228769) is available from GenBank, but its name was erroneously corrected to Burhinus grallarius. How did that happen? It happened because the same scientific name magnirostris was given to the Beach and Bush Stone-Curlews, and two centuries later, it is still causing confusion!
- Double-striped Thick-knee, Hesperoburhinus bistriatus
- Peruvian Thick-knee, Hesperoburhinus superciliaris
- Bush Stone-Curlew, Burhinus grallarius
- Great Stone-Curlew, Esacus recurvirostris
- Beach Stone-Curlew, Esacus neglectus
- Spotted Thick-knee, Oedicnemus capensis
- Water Thick-knee, Oedicnemus vermiculatus
- Eurasian Stone-Curlew, Oedicnemus oedicnemus
- Senegal Thick-knee, Oedicnemus senegalensis
- Indian Stone-Curlew, Oedicnemus indicus
Charadrii Huxley, 1867
The Charadrii and Chioni split dates to about 51 mya. Based on Kuhl et al. (2021) and Černý and Natale (2022), the next split is between Pluvianida (the Egyptian Plover) and the rest of Charadrii. Kuhl et al. dated the split to around 43 mya, which would probably be about 45 mya by the Stiller et al. dating, had they included the Egyptian Plover in their analysis.
Pluvianida: Egyptian Plover Informal?
The Egyptian Plover or Crocodile-bird is sister to all of the remaining Charadrii.
Pluvianidae: Egyptian Plover Reichenbach, 1848
1 genus, 1 species Not HBW Family
Although it is sometimes put in its own family, the Egyptian Plover was typically considered a member of the Glareolidae (pratincoles and coursers). DNA changed everything here. Ericson et al. (2003a), Baker et al. (2007), and Fain and Houde (2007) made clear the Egyptian Plover is not close to the Glareolidae. All three found it to be basal in the Charadrii (in our sense), as did Kuhl et al. (2021) and Černý and Natale (2022, Figs. A.31-A.34). Further, all found it to be sister to the remaining Charadrii, which justifies placing it not just in its own family, but even in its own parvorder (Pluvianida).
- Egyptian Plover / Crocodile-bird, Pluvianus aegyptius
Charadriida Huxley, 1867
The parvorder Charadriida consists of two clades, which I rank as superfamilies: Haematopodoidea and Charadrioidea. Stiller et al. (2024) date the split between them at around 32 mya.
Haematopodoidea Billberg, 1828
The superfamily Haematopodoidea contains three families: Recurvirostridae (Stilts and Avocets), Ibidorhynchidae (Ibisbill), and Haematopodidae (Oystercatchers). Calibration point 12 of Černý and Natale (2022) marks the split between the Recurvirostridae and the rest. They put it at 23 mya, and the split between the Ibisbill and Oystercatchers at about 22.5 mya. Stiller et al. (2024) only date the latter, at 20 mya.
All three groups pass both parts of the family test. Each originated in the family band and each is distinctive. So I rank them as families; Both Stiller et al. (2024) and Černý and Natale (2022) support the family arrangement here, with the Ibisbill sister to the Oystercatchers.
I've corrected the attribution of Recurvirostridae and Haematopodidae. I now cite Billberg, 1828. See Laurent Raty's post on BirdForum, which includes links to both original descriptions. Since the names of both families have equal priority, a first reviser action is required to decide whether the superfamily containing them should be called Recurvirostroidea or Haematopodoidea. Cracraft (1981) may be it. As Raty noted, he put both families in the superfamily Haematopodoidea.
Recurvirostridae: Stilts, Avocets Billberg, 1828
3 genera, 9 species HBW-3
 |
| Click for Recurvirostridae species tree |
|---|
The current arrangement is based on Černý and Natale, (2022). The Banded Stilt, Cladorhynchus leucocephalus, is the basal member of the family Recurvirostridae, which has been arranged accordingly.
Baker et al. (2007) gave a different arrangement of the stilt and avocet genera. However, this seems to be driven by an ND2 sequence for Recurvirostra americana that appears to belong to a Painted-snipe. See Raty's comments on BirdForum for more details. This sort of thing happens more than you might suspect.
- Banded Stilt, Cladorhynchus leucocephalus
- Pied Avocet, Recurvirostra avosetta
- Red-necked Avocet, Recurvirostra novaehollandiae
- American Avocet, Recurvirostra americana
- Andean Avocet, Recurvirostra andina
- Black-necked Stilt, Himantopus mexicanus
- Black-winged Stilt, Himantopus himantopus
- White-headed Stilt, Himantopus leucocephalus
- Black Stilt, Himantopus novaezelandiae
Ibidorhynchidae: Ibisbill Bonaparte 1856
1 genus, 1 species HBW-3
Kuhl et al. (2021) found the distinctive Ibisbill sister to the Haematopodidae (oystercatchers), as did Černý and Natale (2022). They discussed it in and around Figures 3--5.
- Ibisbill, Ibidorhyncha struthersii
Haematopodidae: Oystercatchers Billberg, 1828
2 genera, 13 species HBW-3
 |
| Click for Haematopodidae species tree |
|---|
The arrangement of the oystercatchers here is based on material from a presentation by Senfeld et al. (2020b). You'll notice that it differs a bit from A.31 and A.33 in Černý and Natale (2022). One thing they do agree on is the division into two deeply separated clades corresponding to Old and New World Oystercatchers. I have placed the New World species in the genus Prohaematopus (Mathews 1913, type ater).
Far Eastern Oystercatcher, Haematopus osculans: Based on Senfeld et al. (2020b), the Far Eastern Oystercatcher, Haematopus osculans, has been split from the Eurasian Oystercatcher, Haematopus ostralegus.
I continue to list the extinct Canary Islands Oystercatcher, Haematopus meadewaldoi, as a separate species in spite of Senfeld et al.'s (2020a) recommendation to merge it into the Eurasian Oystercatcher, Haematopus ostralegus. See Collar et al. (2021) for details. The fact that the taxa appear to have been acting as biological species makes this a very different case than species such as the Redpoll.
- Magellanic Oystercatcher, Prohaematopus leucopodus
- Blackish Oystercatcher, Prohaematopus ater
- Black Oystercatcher, Prohaematopus bachmani
- American Oystercatcher, Prohaematopus palliatus
- Sooty Oystercatcher, Haematopus fuliginosus
- African Oystercatcher, Haematopus moquini
- Eurasian Oystercatcher, Haematopus ostralegus
- †Canary Islands Oystercatcher, Haematopus meadewaldoi
- Far Eastern Oystercatcher, Haematopus osculans
- Pied Oystercatcher, Haematopus longirostris
- Chatham Oystercatcher, Haematopus chathamensis
- Variable Oystercatcher, Haematopus unicolor
- South Island Oystercatcher, Haematopus finschi
Charadrioidea: Plovers Leach, 1820
There are two families in the superfamily Charadrioidea: Pluvialidae (golden-plovers) and Charadriidae (plovers and lapwings).
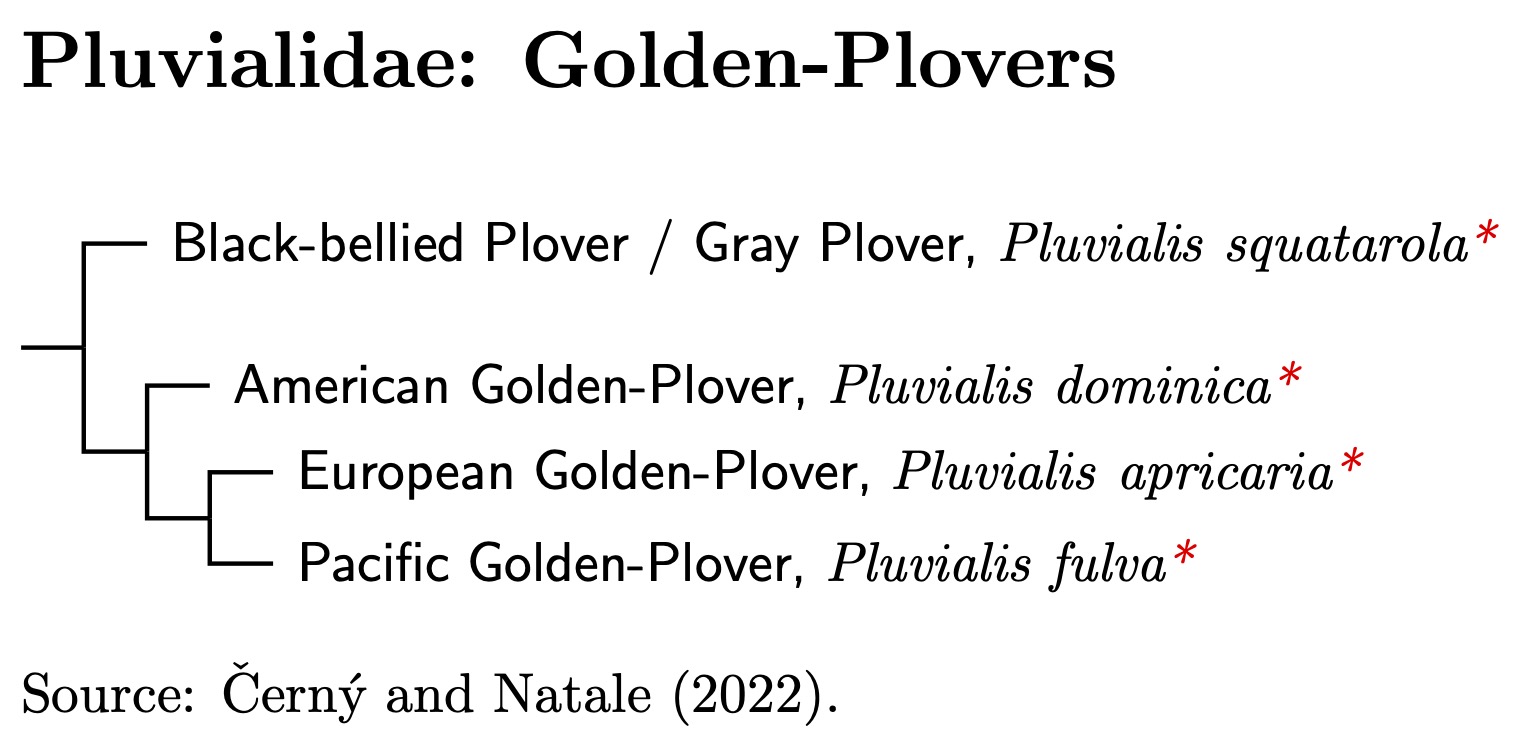
Pluvialidae: Golden-Plovers Wood, 1836
1 genus, 4 species Not HBW Family
One big surprise to come out of the molecular data is that the golden-plovers (and Black-bellied Plover) are not that closely related to the rest of the plovers. Several papers have even suggested that golden-plovers are actually closer to Haematopodoidea, the stilts, avocets, oystercatchers, and ibisbill (Ericson et al., 2003a; Baker et al., 2007; Fain and Houde, 2007; Dufour et al., 2024). Figure A.31 of Černý and Natale (2022) puts them in a more basal position, sister to Charadriidae plus Haematopodoidea.
Not all agreed. Baker et al. (2012) found the golden-plovers sister to Charadriidae and argued that results showing otherwise were artifacts of the DNA used. They argued that rapidly changing mitochondrial DNA was skewing the results, and that the nuclear DNA used was changing too slowly to overcome that. They included additional nuclear DNA chosen to provide a stronger signal, and it changed the result. Figure A.33 of the supplement to Černý and Natale (2022) supports this. See also Figures 3--5 of Černý and Natale (2022) and the related text where Pluvialis and other taxa with inconsistent placement are considered.
The more radical suggestions are most likely incorrect. Rather, the golden-plovers seem to be sister to the main group of plovers, Charadriidae.
Stiller et al. (2024) do not include ahy of the Pluvialis plovers in their analysis. Černý and Natale (2022) estimate that the common ancestor of the golden-plovers and other plovers lived shortly after the split between the Haematopodoidea and Charadriodea. Using the Stiller et al. (2024) timeline, that translates to about 30 mya. That time depth makes it more than reasonable to treat the distinctive golden-plovers as a separate family.
 |
| Golden-Plover tree |
|---|
- Black-bellied Plover / Gray Plover (Pluvialis squatarola)
- American Golden-Plover (Pluvialis dominica)
- European Golden-Plover (Pluvialis apricaria)
- Pacific Golden-Plover (Pluvialis fulva)
Charadriidae: Plovers, Dotterels, Lapwings Leach, 1820
19 genera, 65 species HBW-3
 |
| Click for Charadriidae species tree |
|---|
As mentioned above, the split between the Pluvialidae and Charadriidae ocurred about 30 mya. The crown group of Charadriidae is a bit younger. Stiller et al. (2024) found that the common ancestor of the Killdeer and Kentish Plover lived about 24 mya. The crown group must be older than that. Černý and Natale (2022) estimate that Oreopholus split about a million years earlier, so we take the crown group to be about 25 million year old. In contrast, Černý and Natale directly estimated the crown group's age at 33 million years.
The last two editions of the Howard and Moore Checklist (Dickinson, 2003; Dickinson and Remsen, 2013) divided the plovers into three subfamilies: Pluvialinae, Vanellinae, and Charadriinae. Moreover, most of the species were in two genera: Vanellus and Charadrius.
Problems with that classification were already apparent in Christian et al. (1992). These problems intensified with Joseph et al. (1999). Both demonstrated that Charadriinae and Charadrius were not natural groups. In retrospect, we can even see the current TiF organization starting to appear in Joseph et al. (1999).
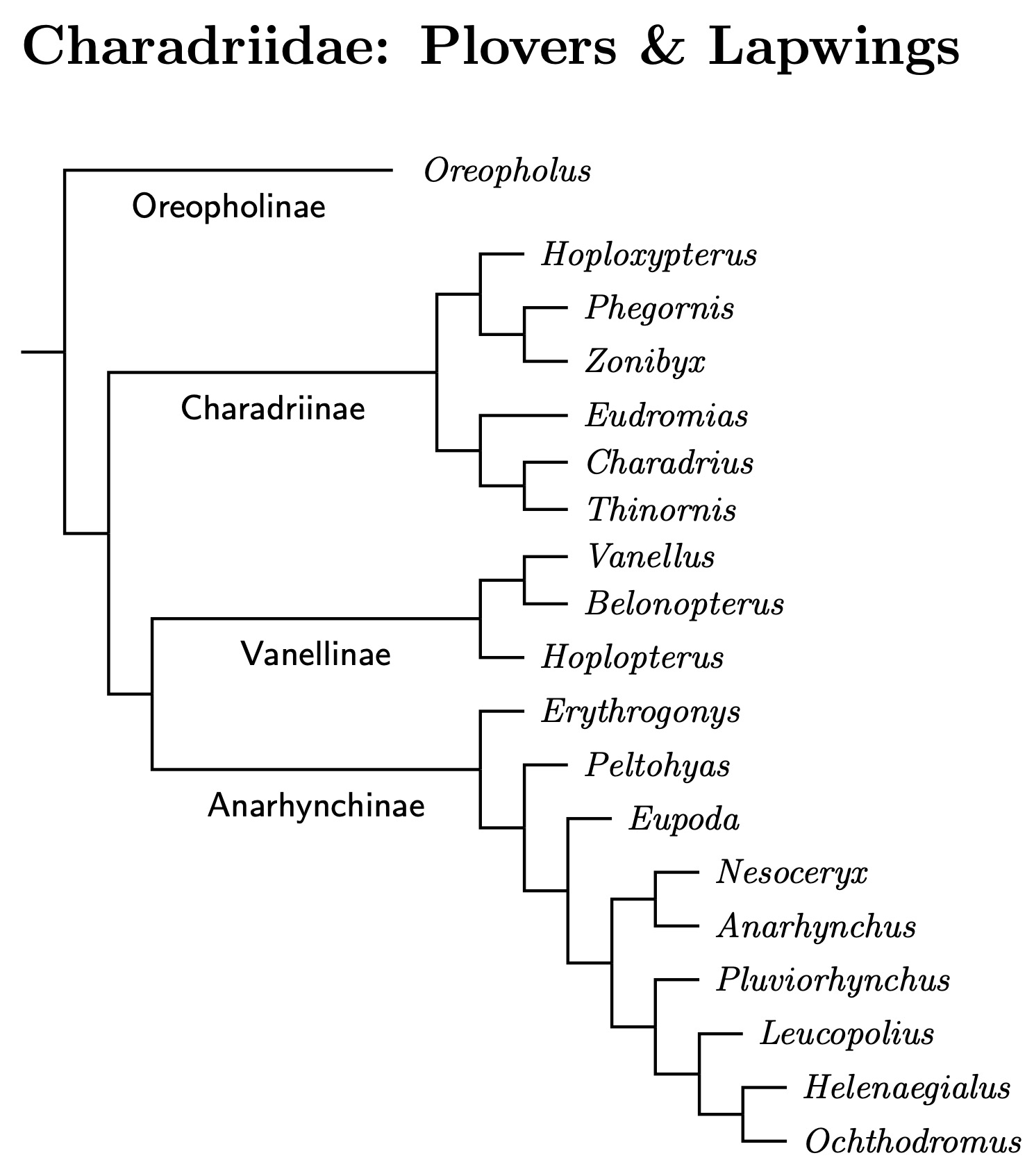
Charadriidae à la TiF
The organization here is rather different than the three subfamily model. Pluvialinae has been removed from Charadriidae and promoted to its own family. The latest TiF reorganization of the plovers remaining in Charadriidae retains Vanellinae and divides Charadriinae into three non-sister subfamilies. We have:
- Oreopholinae, which contains a single species, the Tawny-throated Dotterel, Oreopholus ruficollis
- Charadriinae, with 6 genera, including Charadrius and Thinornis. Charadrius is now restricted to 4 species.
- The lapwing subfamily Vanellinae.
- The remaining plovers are in Anarhynchinae, divided into 9 genera. It includes the Sand-Plovers in Eupoda, a clade of former Kentish Plovers (Leucopolius), a small group of related plovers centered on Africa (Helenaegialus), and the remaining plovers from the Americas (Ochthodromus).
Two of those subfamilies are at least 24 million years old, and the other two are perhaps a million years younger. This suggests we might elevate them all to family rank. However, except for the lapwings, all of the species involved are rather similiar, so I prefer to leave them all as subfamilies of a single family, Charadriidae.
Since the 2021 updates, I've rethought the situation with The Pied Lapwing, Hoploxypterus cayanus. It seems it's not a lapwing. However, we don't have clarity on what it is. Černý and Natale (2022) give two options: sister to Zonibyx and Phegornis (A.31) or sister to a combined Vanellinae and Anarhynchinae (A.33), while Dufour et al. (2024, Fig. S1) found it sister to Oreopholus. This problem is highlighted in Figure 4 of Černý and Natale (2022), where it is referred to as 'Vanellus' cayanus.
There's no clarity, but I have to choose, and I've put the Pied Lapwing (or Plover) sister to the Diademed Sandpiper-Plover, Phegornis mitchellii and Rufous-chested Dotterel (or Plover), Zonibyx modestus, as in Figure A.31 of Černý and Natale (2022).
We now consider the subfamily Charadriinae. Within Charadriinae, the Rufous-chested Dotterel (or Plover) is sister to the Diademed Sandpiper-Plover. They seem to be only distant relatives, and I previously put the Sandpiper-Plover in the monotypic genus Zonibyx (Reichenbach, 1852). These sister taxa are basal in Charadriinae. The Eurasian Dotterel, Eudromias morinellus is the next branch.
This is followed by the true Charadrius plovers, consisting of 4 species: Killdeer (vociferus), Common Ringed Plover (hiaticula), Semipalmated Plover (semipalmatus), and Piping Plover (melodus). Interestingly, birds such as the Kentish Plover and its relatives are not even in the same subfamily.
Thinornis Revisited: Previously, I tried to piece together a reasonable phylogeny for the Thinornis clade. But there was a problem, too many phylogenies that weren't easily reconciled. This has been fixed by Černý and Natale (2022), which provides a reasonable phylogeny in (A.31 and A.33).
I've put the Thinornis clade all in one genus: Thinornis, G.R. Gray 1845, type novaeseelandiae. That means I merged Afroxyechus, "Afroxyechus", and "Thinornis" into Thinornis. The name Thinornis has priority over Afroxyechus, Mathews 1913, type tricollaris as well as two other Mathews names from 1913, Elseyornis (type melanops) and Paroxyechus (type placidus).
The remaining plovers divide into two subfamilies, the lapwings (Vanellinae) and the remaining plovers (Anarhynchinae). Although nine of the 24 lapwings have been sequenced, the diversity of plumage and form among the lapwings makes it hard to construct a reliable phylogeny. They are so diverse that 80% are the type species of some genus.
As mentioned above, I've removed the Pied Lapwing, Hoploxypterus cayanus from the lapwing subfamily. It seems it is not actually a lapwing. The presumed basal group in the Vanellinae consists of the Northern, Southern, and Andean Lapwings, and that group seems sister to the remaining lapwings. The division between the Northern Lapwing and the Southern and Andean Lapwings appears deep, so I use three genera for the lapwings:
- Vanellus is restricted to the Northern Lapwing
- Southern Lapwing and Andean Lapwing are transferred to Belonopterus (Reichenbach 1852, type chilensis)
- Until we know more, the other 20 lapwings have been placed in Hoplopterus (Bonaparte 1831, type spinosus). I did not find the total evidence tree useful here as it contradicts the available genetic data. I retained the previous arrangement, with the above genera pulled out as above.
The last plover subfamily is Anarhynchinae. The Red-kneed Dotterel, Erythrogonys cinctus and Inland Dotterel, Peltohyas australis are the first two basal branches, in that order. The other species were all once in Charadrius. I treat them as five clades, and the divisions within one of those clades seem to be relatively deep, giving us a total of seven genera.
Unfortunately, the plovers are only loosely calibrated by Černý and Natale (2022). As a result, the ages they show on Figure 6 for the various clades I'm calling genera reflect how the calibration estimator spreads out time rather than closely reflecting reality. Better estimation could change the genus boundaries.
The first of the five branches is the sand-plover clade, Eupoda (J.F. Brandt 1845, type asiatica). Based on Wei et al. (2022), the Lesser Sand-Plover has been split into two species:
- Tibetan Sand-Plover, Eupoda atrifrons, which includes pamirensis and schaeferi and
- Siberian Sand-Pover, Eupoda mongola, including stegmanni.
Interestingly, the Siberian and Greater Sand-Plovers are sister species, and the Tibetan Sand-Plover is sister to the pair, but not directly sister to the Siberian, with which it was formerly considered conspecific.
The sand-plovers are followed by three species I previously put in genus Anarhynchus. These are now on two separate branches, and the branch containing two is relatively deeply divided, so I've split them into three genera. That gives us
- The Double-banded Plover, Anarhynchus bicinctus, is now in the monotypic genus Nesoceryx (Mathews, 1920).
- The Wrybill, which remains Anarhynchus frontalis (Quoy and Gaimard, 1830).
- The New Zealand Plover, Anarhynchus obscurus, is now in the monotypic genus Pluviorhynchus (Bonaparte 1856).
I had previously moved the remaining plovers from Charadrius to Ochthodromus (Reichenbach 1852, type wilsonia). Although some uncertainty, there appear to be three clades in Ochthodromus. It seems appropriate to separate them as genera based on both biogeography. I consider the age of the various genera in Charadriidae to be uncertain.
- Leucopolius (Bonaparte 1856, type marginatus) includes the Kentish Plover, Leucopolius alexandrinus, and close relatives
- Helenaegialus (Mathews 1913, type sanctaehelenae) includes thoracicus, pecuarius, and sanctaehelenae.
- Ochthodromus (Reichenbach 1852, type wilsonia). Besides Wilson's (wilsonia) and Collared Plovers (collaris), it includes the Mountain Plover (montanus) and Puna (alticola) and Two-banded Plovers (falklandicus).
The status of taxa in the Kentish-Snowy Plover complex has been controversial. Küpper et al. (2009) found that the Kentish, Snowy, and White-fronted Plovers represented independent groups, with little or no evidence of gene flow between them. Not only does this separation appear to be long-standing, but the White-fronted Plover seems to be more closely related to the Kentish Plover than to the Snowy Plover. Accordingly, the Kentish (L. alexandrinus) and Snowy (L. nivosus) Plovers are treated as separate species below.
Rheindt et al. (2011b) provide further evidence in favor of separating Kentish and Snowy Plovers. They also found that the Malaysian Plover, Leucopolius peronii, is a member of the group. It is most closely related to the White-fronted Plover, Leucopolius marginatus. Both are more closely related to the Kentish Plover than to the Snowy Plover.
The White-faced Plover, Leucopolius dealbatus, differs from the Kentish Plover ecologically and in size and plumage (Bakewell and Kennerly, 2008; Kennerly et al., 2008). Whether it is a separate species or just some form of Kentish Plover has remained a question. However, Sadanandan et al. (2019) found only limited hybridization, suggesting it is a separate species. See also Wang et al. (2019). The HBW Illustrated Checklist (del Hoyo and Collar, 2014) recognizes it as a separate species, and I also now recognize it as such.
DNA Issues
Long-billed Plover, Thinornis placidus: There has been an issues with DNA from the Long-billed Plover, Thinornis placidus. A Kentish Plover gene (labelled placidus) has been mixed in with the downloaded DNA sequences, creating an electronic chimera. See Päckert (2021) and Sangster and Luksenburg (2021) for more on this and other issues with published and archived genes. It looks like this also affects Dufour et al. (2024, Fig. S1). This was fixed in the published version of Černý and Natale (2022).
Caspian Plover, Eupoda asiatica: There had been a problem with DNA problem involvimg the Caspian Plover, Eupoda asiatica. It seems that chimeric data was used in the Černý and Natale preprint. This was corrected in Černý and Natale (2022), who now find it sister to the Oriental Plover, Eupoda veredus. Dufour et al. (2024, Fig. S1) apparently also used the chimeric data.
The Wrybill, frontalis, New Zealand Plover, obscurus, and Double-banded Plover, bicinctus form a clade in Dos Remedios et al. (2015) and Barth et al. (2013). However, Černý and Natale (2022) disagree, giving two other variants in Supplementary Figures A.31 and A.33. In A.31, which I follow here, the New Zealand Plover, obscurus, is on a separate and adjacent branch. The HBW Illustrated Checklist (del Hoyo and Collar, 2014) recognizes the two subspecies of the New Zealand Plover as separate species.
Which Scientific Name?
Hooded Dotteral: cucullatus or rubricollis:? One point to clarify before considering the phylogeny involves the Hooded Dotteral. If you look at the papers cited, you'll notice that some refer to the Hooded Dotteral as rubricollis rather than cucullatus. There are two competing names, Charadrius rubricollis Gmelin, 1789 and Charadrius cucullatus Vieillot, 1818. Obviously, rubricollis is older, so why is cucullatus used?
The answer is in Mcallan and Christidis (1998) and Olson (1998). The short version of the story is that Gmelin's name rubricollis is based on Latham's description of a bird he called the Red-necked Plover, an odd name for the Hooded Dotterel, which doesn't have a red neck. Latham's description seems to have been based on drawings by Ellis from Cook's third voyage, but apparently he conflated drawings of the Dotterel and a Red-necked Phalarope! Mcallan and Christidis. Mcallan and Christidis attempted to correct this, but failed to correctly follow the ICZN Code. Olson corrected their correction, putting rubricollis among the synonyms of the Red-necked Phalarope. This automatically makes cucullatus the name of the Hooded Dotterel.
Oreopholinae Informal
- Tawny-throated Dotterel, Oreopholus ruficollis
Charadriinae Leach, 1820
- Pied Lapwing / Pied Plover, Hoploxypterus cayanus
- Diademed Sandpiper-Plover, Phegornis mitchellii
- Rufous-chested Dotterel / Rufous-chested Plover, Zonibyx modestus
- Eurasian Dotterel, Eudromias morinellus
- Killdeer, Charadrius vociferus
- Common Ringed Plover, Charadrius hiaticula
- Semipalmated Plover, Charadrius semipalmatus
- Piping Plover, Charadrius melodus
- Hooded Dotterel, Thinornis cucullatus
- Black-fronted Dotterel, Thinornis melanops
- Shore Dotterel, Thinornis novaeseelandiae
- Little Ringed Plover, Thinornis dubius
- Long-billed Plover, Thinornis placidus
- Forbes's Plover, Thinornis forbesi
- Three-banded Plover, Thinornis tricollaris
Vanellinae: Lapwings Bonaparte, 1842
- Northern Lapwing, Vanellus vanellus
- Southern Lapwing, Belonopterus chilensis
- Andean Lapwing, Belonopterus resplendens
- Long-toed Lapwing, Hoplopterus crassirostris
- Blacksmith Lapwing, Hoplopterus armatus
- Spur-winged Lapwing, Hoplopterus spinosus
- River Lapwing, Hoplopterus duvaucelii
- Black-headed Lapwing, Hoplopterus tectus
- Yellow-wattled Lapwing, Hoplopterus malarbaricus
- White-crowned Lapwing, Hoplopterus albiceps
- Senegal Lapwing, Hoplopterus lugubris
- Black-winged Lapwing, Hoplopterus melanopterus
- Crowned Plover / Crowned Lapwing, Hoplopterus coronatus
- African Wattled Lapwing, Hoplopterus senegallus
- Spot-breasted Lapwing, Hoplopterus melanocephalus
- Brown-chested Lapwing, Hoplopterus superciliosus
- Gray-headed Lapwing, Hoplopterus cinereus
- Red-wattled Lapwing, Hoplopterus indicus
- †Javan Lapwing, Hoplopterus macropterus
- Banded Lapwing, Hoplopterus tricolor
- Masked Lapwing, Hoplopterus miles
- Sociable Lapwing, Hoplopterus gregarius
- White-tailed Lapwing, Hoplopterus leucurus
Anarhynchinae Baird, Brewer and Ridgway, 1884
- Red-kneed Dotterel, Erythrogonys cinctus
- Inland Dotterel, Peltohyas australis
- Caspian Plover, Eupoda asiatica
- Oriental Plover, Eupoda veredus
- Tibetan Sand-Plover, Eupoda atrifrons
- Greater Sand-Plover, Eupoda leschenaultii
- Siberian Sand-Plover, Eupoda mongola
- Double-banded Plover, Nesoceryx bicinctus
- Wrybill, Anarhynchus frontalis
- New Zealand Plover, Pluviorhynchus obscurus
- Red-capped Plover, Leucopolius ruficapillus
- Snowy Plover, Leucopolius nivosus
- Chestnut-banded Plover, Leucopolius pallidus
- White-fronted Plover, Leucopolius marginatus
- Malaysian Plover, Leucopolius peronii
- White-faced Plover, Leucopolius dealbatus
- Kentish Plover, Leucopolius alexandrinus
- Javan Plover, Leucopolius javanicus
- Madagascan Plover, Helenaegialus thoracicus
- Kittlitz's Plover, Helenaegialus pecuarius
- St. Helena Plover, Helenaegialus sanctaehelenae
- Mountain Plover, Ochthodromus montanus
- Wilson's Plover, Ochthodromus wilsonia
- Collared Plover, Ochthodromus collaris
- Puna Plover, Ochthodromus alticola
- Two-banded Plover, Ochthodromus falklandicus
Limicoli Garrod, 1873
There are two names in use for this group. The term Scolopaci (Strauch 1978?) seems to be the current fashion. However, Limicoli has a lot of priority and a long history of use (often as Limicolae). The code does not extend to ordinal-level names. This means either is allowed, in spite of clear priority for Limicoli. Stiller et al. (2024) dated the split between the Limicoli and Turnici at about 51 mya, but I have bumped it up to 53 mya because of the changes to correct a misdating of the alcids, as revealed by a close study of the calibrations in Černý and Natale (2022).
Limicoli is divided into two parvorders, Parrida and Limicolida. The split dates to about 41 mya (Stiller et al., 2024). Limicolida contains a single family, Scolopacidae, while the higher taxonomy of Parrida is more complex. The change in name to Scolopacoidea and is due to the fact that priority at the order-group and family-group levels works independently.
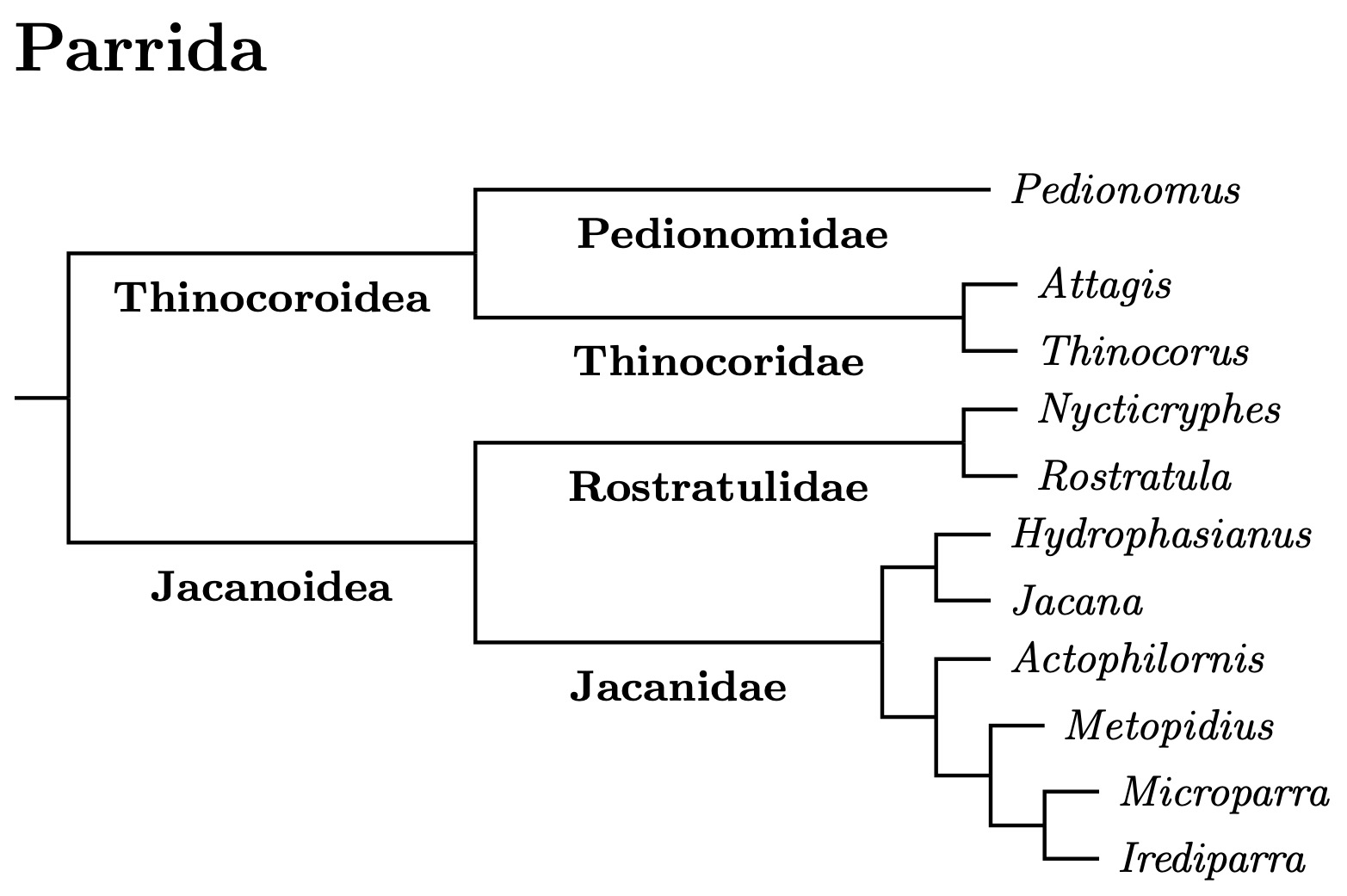
Parrida Sharpe, 1891
The name Parrida was introduced by Sharpe (1891) as suborder Parrae in a paper on classification. If you wonder why he called it Parrae, it is enough to realize that Linnaeus called the Wattled Jacana Parra jacana. You also see echoes of this in the jacana genus names Microparra and Irediparra. The modern ending for an avian parvorder is ‑ida, so it becomes Parrida.
Unlike the names of family and genus level groups, names of ordinal level groups are not regulated by the International Commission on Zoological Names. Nonetheless, I continue to follow the principle of priority whenever possible. As far as I can tell, Sharpe was the first to name this group.
Parrida consists of 4 small families totalling 16 species. In spite of the small number of species, the Parrida are further divided into two superfamilies! The Parrida are sister to the much larger group of sandpipers, which are ranked as both a superfamily (Scolopacoidea) and family (Scolopacidae). The divisions between the small groups contained in Parrida are deep enough for family recognition. Moreover, each family in Parrida is easily distinguishable.
 |
| Click for Parrida family and genus tree |
|---|
The analysis in Stiller et al. (2024) contains members of each Parrida family. They date the split between the Parrida and Scolopacoidea at about 41 million years ago (Černý and Natale estimated 43 mya). The Parrida divided into the superfamilies Jacanoidea and Thinocoroidea about 34.5 million years ago.
Thinocoroidea Sundevall, 1836
Thinocoroidea consists of the monotypic plains-wanderer family (Pedionomidae) and the seedsnipe family (Thinocoridae). The two families seem to have separated around 25 million years ago, in the Oligocene.
Pedionomidae: Plains-wanderer Bonaparte, 1856
1 genus, 1 species HBW-3
- Plains-wanderer, Pedionomus torquatus
Thinocoridae: Seedsnipe Sundevall, 1836
2 genera, 4 species HBW-3
- Rufous-bellied Seedsnipe, Attagis gayi
- White-bellied Seedsnipe, Attagis malouinus
- Gray-breasted Seedsnipe, Thinocorus orbignyianus
- Least Seedsnipe, Thinocorus rumicivorus
Jacanoidea Chenu & des Murs, 1854 (1840)
Like the Thinocoroidea, the Jacanoidea originated about 34 million years old. The two included families, the painted-snipe (Rostratulidae) and jacanas (Jacanidae) seem to have separated in the Oligocene, around 30.5 million years ago (Černý and Natale, 2022).
Rostratulidae: Painted-snipe Mathews, 1913-14 (1855)
2 genera, 3 species HBW-3
- South American Painted-snipe, Nycticryphes semicollaris
- Greater Painted-snipe, Rostratula benghalensis
- Australian Painted-snipe, Rostratula australis
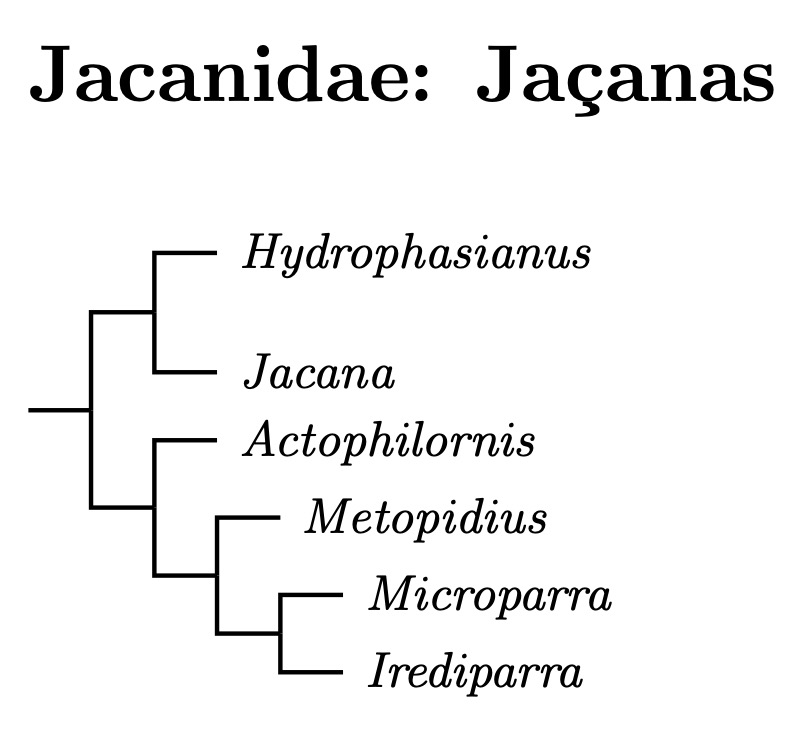
Jacanidae: Jacanas Chenu & des Murs, 1854 (1840)
6 genera, 8 species HBW-3
 |
| Click for Jacanidae species tree |
|---|
Whittingham et al. (2000) found two clades of Jacanas, one containing Hydrophasianus and Jacana, the other consisting of the other four genera. These two groups could be treated as subfamilies, but with only 8 species of Jacana, doing so seems to be taxonomic overkill.
Černý and Natale (2022) give two different phylogenies (Figures A.31 and A.33), which differ in the sequencing of the last four genera. Neither matches the 2021 version of the TiF tree. Both treat the two Actophilornis as sister species, and also Microparra and Irediparra. The only real question is where to put Metopidius. I've somewhat arbitrarily gone with Supplemental Figure A.31 from Černý and Natale (2022)
- Pheasant-tailed Jacana, Hydrophasianus chirurgus
- Northern Jacana, Jacana spinosa
- Wattled Jacana, Jacana jacana
- African Jacana, Actophilornis africanus
- Madagascan Jacana, Actophilornis albinucha
- Bronze-winged Jacana, Metopidius indicus
- Lesser Jacana, Microparra capensis
- Comb-crested Jacana, Irediparra gallinacea
Limicolida Garrod, 1873
The parvorder Limicolida originated around 41 million years ago. It contains only one extant superfamily, Scolopacoidea, and one family Scolopacidae. Both Limicolida and Scolopacidae are based on taxa in the family Scolopacidae. The name change is due to a combination of priority and the fact that the ICZN code does not apply to names above family level. Maybe that will be changed someday, but today is not that day.
Scolopacoidea Rafinesque, 1815
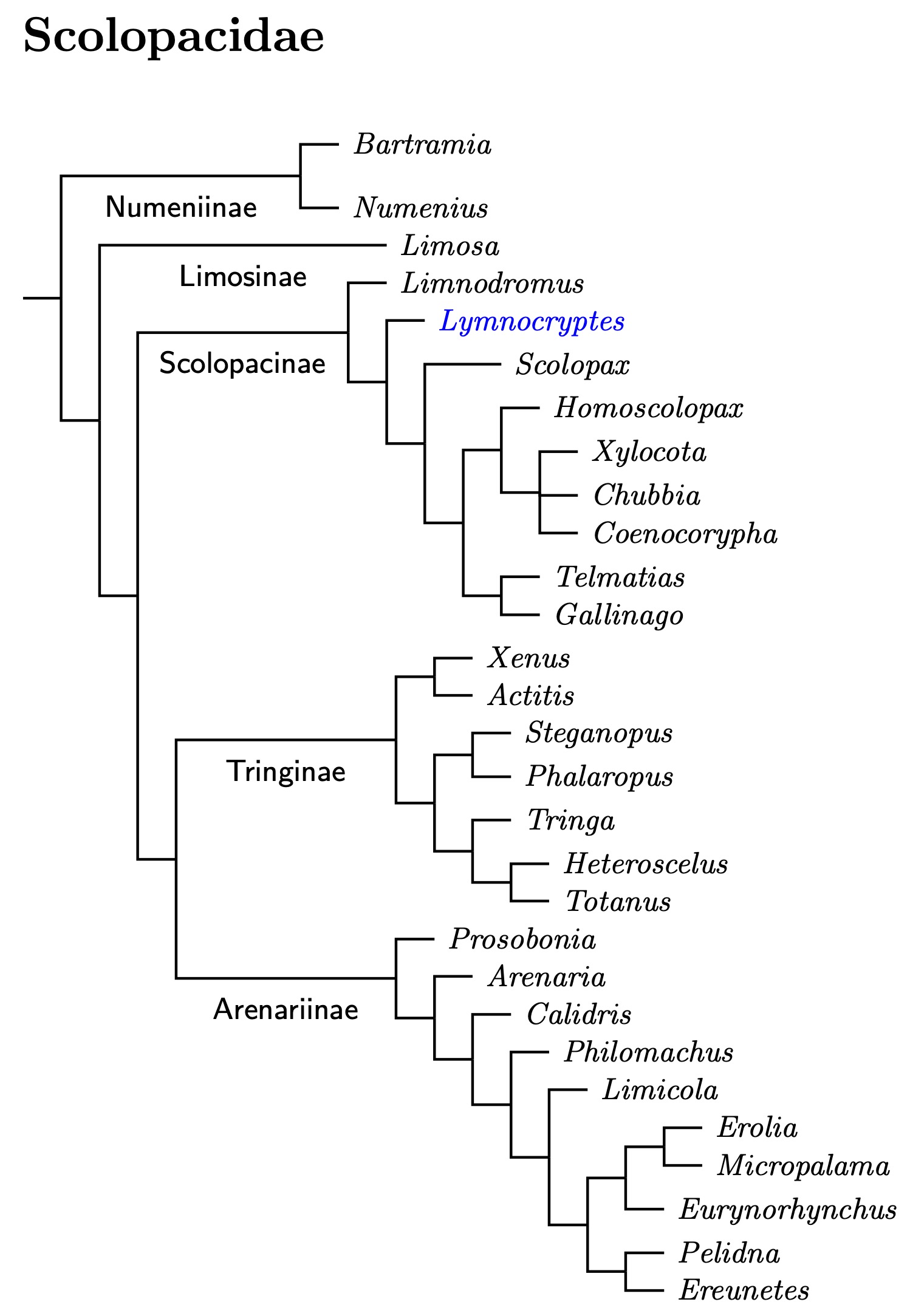
The superfamily Scolopacoidea is presumably younger than Limicolida's 41 million years, but I don't know where to draw the line. Scolopacoidea currently contains one extant family, Scolopacidae, which is divided into 5 subfamilies: Numeniinae (curlews), Limosinae (godwits), Scolopacinae (dowitchers and snipe), Tringinae (phalaropes and shanks), and Arenariinae (turnstones and stints).
Stiller et al. (2024) dated the separation between Limosa and the clade Arenariinae (containing Calidris and Arenaria) at 27 mya. Using the TiF phylogeny (Dufour et al., 2024, Fig. S1 is different), that clade contains the subfamilies Scolopacinae, Tringinae, and Arenariinae. If this age is correct we should considre promoting some or even all of the Scolopacidae subfamilies to families. That is, the curlews and Upland Sandpiper, the godwits, the dowitchers and snipe, the phalaropes and shanks, and the turnstones and stints, might all qualify as separate families within the superfamily Scolopacoidea.
I'm not convinced that is a good idea. I think there is a lot to be said for emphasizing the unity of the sandpipers and using subfamilies to separate the various groups. Also, the age might be an artifact of the estimator used to incorporate calibration points. Look at the shape of portion of the Scolopacinae downstream of calibration point 8 in Černý and Natale (2022). Based on an examination of other calibrated trees, I suspect that without it, the Scolopacinae downstream of point 8 would be stretched out to look more like Limnodromus, Limnocryptes, and Scolopax.
Scolopacidae: Sandpipers, Snipe Rafinesque, 1815
30 genera, 99 species HBW-3
 |
| Click for Scolopacidae species tree |
|---|
The overall treatment of the sandpipers is based on Černý and Natale (2022). Although some details may differ, it is generally consistent with the results in Baker et al. (2007), Ericson et al. (2003a), Fain and Houde (2007), Gibson (2010), Gibson and Baker (2012), Paton et al. (2003), Pereira and Baker (2005), and Thomas et al. (2004a) as well the more heterogeneous evidence assembled by Thomas et al. (2004b).
Černý and Natale (2022) devote 3 calibration points to the Scolopacidae (#7, #8, and #9). None impose tight constraints on ages, neither for Černý and Natale nor Stiller et al. (2024). Stiller et al. only give ages for the Limosa split and the Arenaria-Philomachus split.
Families or Subfamilies?
Using the Černý and Natale age estimated, adjusted to the Stiller et al. scale. This puts the crown group of Scolopacidae (defined by the Numeniinae split) at 32.5 mya. The godwits (Limosinae) split at 27 mya, followed by the dowitchers, snipe, and woodcock (Scolopacinae) at 25.7 mya. Finally, the split between the phalaropes and shanks (Tringinae), and turnstones and stints (Arenariinae) happened around 23 mya. This means that each subfamily is old enough to consider for family status.
But are the subfamilies distinctive enough? Although each subfamily is fairly easily recognizable as such, I don't think they are distinctive enough. To me, they don't stand out quite enough from the other Scolopacidae. I prefer to stress their common origin by recognizing the whole clade as a single familiy, rather that as superfamily Scolopacoidea.
Numeninae (curlews)
Numenius: I've rearranged the genus Numenius based on Tan et al. (2023). An earlier paper by Tan et al. (2019), where the “et al.” are partially different, has finally convinced me to split the Whimbrel, Numenius phaeopus, into Eurasian Whimbrel, Numenius phaeopus, and Hudsonian Whimbrel, Numenius hudsonicus. Tan et al. found no evidence of gene flow between the American Whimbrels and those in far eastern Siberia (variegatus). The subspecies variegatus is not composed of hybrids Whimbrels.
This means that the Hudsonian Whimbrel, Numenius hudsonicus, consists only of the subspecies hudsonicus and rufiventris. All of the other subspecies — islandicus, phaeopus, alboaxillaris, rogachevae, and variegatus — belong to the Eurasian Whimbrel, Numenius phaeopus.
Timing Issues: Tan et al. also estimated the Numenius clade's age at approximately 5 million years. This contrasts dramatically with Černý and Natale's (2022) estimate of approximately 17 million years (or about 12½ mya, expressed using the Stiller et al. scale). I suspect the true value is younger than that, and that Černý and Natale's estimate has been dragged into the past by the way the software handles calibration points. I also wonder whether the Bartramia branch is anywhere near 21.7 million years old on the Stiller et al. scale.
This does not mean that I think the estimated age for Scolopacidae should change, only that I think some of the species involved have relatively recent origins. What calibration point 8 did to snipe relative to the woodcocks and dowitchers illustrates my point. I think the woodcock and dowitcher clades should also be similarly compressed toward the present.
Limosinae (godwits)
Limosinae consists of four species of godwit. The taxonomy is straightforward.
Scolopacinae (dowitchers, snipe, & woodcock)
The dowitchers, snipe, and woodcocks (Scolopacinae) are next.
Dowitchers: The split between the Asian Dowitcher and the two American dowitchers looks pretty old in Černý and Natale (2022). In fact, figure 6 shows it at about 29 mya, too old for all the dowitchers to be in the same genus. Rescaling using Stiller et al. (2024) dates reduces the age of the dowitcher split, but it is still old, around 22 mya. That's way too old for all three species to be in the same genus. As a result, I moved the Asian Dowitcher to the monotypic genus Pseudoscolopax Blyth, 1859. The Asian Dowitcher is now Pseudoscolopax semipalmatus.
The Lymnocryptes Problem: What are the Jack Snipe's closest relatives? Baker, Pereira, and Paton (2007) addressed this problem. Their analysis included one species from each of the shorebird genera (two from Turnix) and used four genes: 12S, ND2, cyt-b and RAG-1. Their conclusion was that the Jack Snipe, Limnocryptes minimus was most closely related to the the dowitchers (Limnodromus).
So the matter stood until Černý and Natale's (2021) preprint. They included most of the shorebird species. Suddenly, there was a problem with Lymnocryptes. While one method of analysis (figure A.3, in the appendix) found it sister to the American dowitchers (but not the Asian), the other two (figures A.5 and A.7) gave a surprising result. It grouped with the godwits!
Ultimately, the godwit relation was tracked down to a corrupted DNA sequence that was part Jack Snipe, and part Hudsonian Godwit -- a chimera. They fixed this in the published version, Černý and Natale (2022). But there was another problem with Lymnocryptes. It was closer to the two American dowitchers than to the Asian Dowitcher. Yet one look at the Jack Snipe and the three dowitchers is enough to tell you this can't be right either.
The two situations are illustrated on a tree Laurent Raty posted to BirdForum It is a RAG-1 phylogeny that he constructed from GenBank data. Problematic samples are marked in red.
An analysis of morphological data helps explain why the placement of Lymnocryptes seems so wrong (Černý and Natale, figure A.30). It puts the Jack Snipe sister to a clade containing the true snipe and the woodcocks, but not the dowitchers. Further, the plumage of the Jack Snipe is much like that of many snipe. The snipe has stripes! So does the Jack Snipe.
Figure 6 in Černý and Natale blends both morphology and genetics. The result seems reasonable, with the Jack Snipe sister to the woodcocks.
This is all rather surprising. Usually even a little DNA wins vs. morphology when classifying birds. So what is the problem? The fact that Lymnocryptes is on a long branch could signal problem with the genetic data. Also, figure A.34 indicates very high internode uncertainty. Černý and Natale (2022) suggest that there's just not enough sequence data available for the Jack Snipe. But even eliminating RAG-1 (the problem gene) should still leave enough data to get a reasonable result. So I'm puzzled.
There's still more. Dufour et al. (2024, Fig. S1), using only genetic data, found the Jack Snipe basal in the Scolopacinae. That is, it's sister to the combined dowitchers, snipe, and woodcocks. So which is right? More data will eventually tell us.
Unfortunately, I have a list to update before that happens, and the Jack Snipe needs to be on it. I've chosen another option, putting it sister to the snipe-woodcock clade, to the exclusion of the dowitchers. I've marked it in blue on the tree to indicate substantial uncertainty (a question wasn't enough).
Imperial Snipe: Gibson (2010) and Gibson and Baker (2012) found that Imperial Snipe is more closely related to the New Zealand snipe than to the most of the other snipe. Later, Černý and Natale (2022) discovered that the New Zealand snipe are part of a clade containging the Imperial Snipe and Giant Snipe. However, the phylogeny required those two be put in separate genera. We start by moving the Imperial Snipe to the monotypic genus Homoscolopax Mathews 1913. Thus Imperial Snipe is now Homoscolopax imperialis
Giant Snipe and Chubbia Snipe: I already had reasons to think the Giant Snipe would group with two other snipe, for which the genus Chubbia, Mathews 1913, type stricklandii is appropriate. These are Jameson's Snipe, Chubbia jamesoni and Fuegian Snipe, Chubbia stricklandii. Jameson's Snipe has also been called Andean Snipe. The English name was changed partly to avoid confusion with the Puna Snipe, Gallinago andina. The Imperial Snipe is sometimes included in Chubbia, but the phylogeny requires it have it's own genus.
Laurent Raty's RAG-1 phylogeny is the only DNA clue I have concerning the relationship between the Giant Snipe and Chubbia. In his single-gene analysis, the Giant Snipe is in a trichotomy with the two Chubbia species and the New Zealand Snipes (Coenocorypha).
It may turn out that the Giant Snipe belongs in Chubbia, but it may not. Because of this, I've placed it in Xylocota, Bonaparte 1839 (the name belongs to the nominate subspecies). The name Homoptilura, G.R. Gray 1840 has also been applied to the Giant Snipe. In fact, both names are based on Buffon's illustration of the type. Since Bonaparte's name was proposed first, it gets the nod. It's rather easy to see the illustration is of a Giant Snipe.
New Zealand Snipe: The New Zealand snipe (Coenocorypha) are now considered to include 5 exant and recently extinct species based on Baker et al. (2010) and Worthy et al. (2002). The Snares and South Island snipe are quite closely related, with an estimated divergence time of about 50,000 years. Their status as separate species rests on the lack of an aerial display for the Snares Snipe, as well as genetic and plumage differences. The other Coenocorypha are somewhat more distant relatives.
Gallinago Changes:
-
Černý and Natale (2022) made clear there is a division in
Gallinago that occurred about 10 million years ago. I recognize
this by separating many of the Old World species as genus
Telmatias.
That means the Great Snipe, Gallinago media, Solitary Snipe, Gallinago solitaria, Wood Snipe, Gallinago nemoricola, Swinhoe's Snipe, Gallinago megala, Pin-tailed Snipe, Gallinago stenura, and Latham's Snipe, Gallinago hardwickii have all been transferred to genus Telmatias (Boie 1826, type stenura). - The remaining Gallinago snipe stay in Gallinago. This includes the Madagascan Snipe, Gallinago macrodactyla. Černý and Natale (2022) did not have genetic data on this bird. BOW says its “Structurally similar to G. nigripennis”, so I've rather uncertainly treated them as sister taxa.
- Following SACC, I've split the South American Snipe, Gallinago paraguaiae into Pantanal Snipe, Gallinago paraguaiae and Magellanic Snipe, Gallinago magellanica. See Miller et al. (2019) and SACC Proposal #843.
- The Noble Snipe wasn't originally part of Chubbia, and I think it was a mistake on my part to move it there (I think I just carried it along when I moved Chubbia). I'm returning the Noble Snipe to genus Gallinago, and putting it next to the Puna, Pantanal, Magellanic Snipe (G. andina, paraguaiae, and magellanica). Without genetic data on it, I'm reluctant to assume it is their sister taxon.
Tringinae (phalaropes & shanks)
Tribes in Tringinae: Supplementary Figures A.31 and A.33 in Černý and Natale (2022) are entirely in agreement on the phylogeny of Tringinae. The dates in Tringinae are rather unreliable as there are no nearby calibration points. Ignoring that the calibrated Černý and Natale (2022, Fig. 6) tree differs a bit from the TiF tree, we have two obvious breakpoints in the phylogeny. The phalaropes and Terek Sandpiper form a group, and the Common and Spotted Sandpipers are well-separated from the rest of Tringinae. We call these clades tribes. They are Phalaropodini (Bonaparte 1831), Actitini (Informal 2021), and Tringini (Rafinesque 1815).
Willets: The Willet, Tringa semipalmata, has been split into Western Willet, Tringa inornata, and Eastern Willet, Tringa semipalmata, based on Oswald et al., (2016).
Tringa Split: Černý and Natale (2022) provide a better-calibrated tree than past efforts. There's not a good calibration point near the Tringinae, and I believe ages are substantially over-estimated. Nonetheless, the calibration provides some guidance concerning where to draw genus boundaries. I had previously used three subgenera for Tringa and I'm promoting them to regular genera. As a result,
- Tringa (Linnaeus 1758, type ochropus) is restricted to the Green Sandpiper, Tringa ochropus, and the Solitary Sandpiper, Tringa solitaria
- The two tattlers are transferred to genus Heteroscelus (Baird 1858, type brevipes)
- The remaining Tringa sandpipers are placed in genus Totanus (Bechstein 1803, type totanus).
Arenariinae (turnstone & stints)
Černý and Natale (2022) have a calibration point that somewhat constrains dates for the broad genus Calidris. To me, it suggests dividing the subfamily Arenariinae into three tribes: Prosoboniini (Bonaparte 1850), Arenariini (G.R. Gray, 1840), and Calidrini (Reichenbach 1849). This creates the handy term Calidrine, which can be used to refer to the species formerly in the formerly very broad genus Calidris.
Prosobonia: Cibois et al. (2012) found that Aechmorhynchus and Prosobonia are very closely related, enough so to merge Aechmorhynchus (Coues 1874, type parvirostris) into Prosobonia (Bonaparte 1850, type leucoptera). Černý and Natale (2022) have it as the basal group in Arenariinae, and I have put it in tribe Prosoboniini (Bonaparte, 1850).
Aphriza merged into Calidris: It had long been suspected the Surfbird is close to the knots (e.g., Jehl, 1968). This is exactly what Bororwik and McLennan (1999) found in their DNA tree. Indeed, their results suggest the Surfbird and knots are congeneric. The recent analysis by Gibson (2010) and Gibson and Baker (2012), using additional data, concurred, as did Černý and Natale (2022). Based on this, I've merged Aphriza into Calidris.
Černý and Natale's results also suggest that the broad genus Calidris should be split into a number of genera. I had previously done such a thing, and then undone it after the AOU, BOU, and H&M 4th ed. have all merged all of these species into Calidris, as in Banks (2012).
Well, given the new info, I'm undoing it and then some!
- Calidris (Merrem 1804, type canutus) is restricted to three species: Great Knot, Calidris tenuirostris, Red Knot, Calidris canutus, and Surfbird, Calidris virgata.
- The Ruff, Calidris pugnax, is transferred to Philomachus (Merrem 1804, monotypic).
- Broad-billed Sandpiper, Calidris falcinellus, and Sharp-tailed Sandpiper, Calidris acuminatus are transferred to Limicola (Kaup 1816, type falcinellus).
- Curlew Sandpiper, Calidris ferruginea is transferred to the monotypic genus Erolia (Vieillot 1816).
- Stilt Sandpiper, Calidris himantopus is transferred to the monotypic genus Micropalama (Baird 1858).
- Temminck’s Stint, Calidris temminckii, Long-toed Stint, Calidris subminutus, Red-necked Stint, Calidris ruficollis, and Spoon-billed Sandpiper, Calidris pygmeus are placed in Eurynorhynchus (Nilsson 1821, type pygmeus).
- Dunlin, Calidris alpina, Purple Sandpiper, Calidris maritima, and Rock Sandpiper, Calidris ptilocnemis are transferred to Pelidna (Cuvier 1816, type alpina).
- Buff-breasted Sandpiper, Calidris subruficollis, Sanderling, Calidris alba, Baird's Sandpiper, Calidris bairdii, Little Stint, Calidris minuta, Least Sandpiper, Calidris minutilla, White-rumped Sandpiper, Calidris fuscicollis, Pectoral Sandpiper, Calidris melanotos, Semipalmated Sandpiper, Calidris pusilla, and Western Sandpiper, Calidris mauri are transferred to Ereunetes (Illiger 1811, type pusilla). I had previously put subruficollis in Tryngites (Cabanis 1856), but it groups with the other Ereunetes in Černý and Natale (2022), Supplementary Figures A.31 and A.33 (the total evidence tree, Figure 6, puts it elsewhere, but I don't think the other evidence should override the genetics).
Interestingly, all but one of the Eurasian stints end up in Eurynorhynchus. In contrast, the similarly-sized American peeps are in Ereunetes. The one exception is the Little Stint. It groups with the American peeps. There is some minor taxonomic uncertainty surrounding the ordering of species in the Ereunetes clade.
Numeniinae: Curlews G.R. Gray, 1840
- Upland Sandpiper, Bartramia longicauda
- Little Curlew, Numenius minutus
- Bristle-thighed Curlew, Numenius tahitiensis
- Eurasian Whimbrel, Numenius phaeopus
- Hudsonian Whimbrel, Numenius hudsonicus
- Long-billed Curlew, Numenius americanus
- Eskimo Curlew, Numenius borealis
- Far Eastern Curlew, Numenius madagascariensis
- Slender-billed Curlew, Numenius tenuirostris
- Eurasian Curlew, Numenius arquata
Limosinae: Godwits G.R. Gray, 1841
- Bar-tailed Godwit, Limosa lapponica
- Black-tailed Godwit, Limosa limosa
- Hudsonian Godwit, Limosa haemastica
- Marbled Godwit, Limosa fedoa
Scolopacinae: Dowitchers, Snipe, and Woodcock Rafinesque, 1815
- Asian Dowitcher, Pseudoscolopax semipalmatus
- Short-billed Dowitcher, Limnodromus griseus
- Long-billed Dowitcher, Limnodromus scolopaceus
- Jack Snipe, Lymnocryptes minimus
- Eurasian Woodcock, Scolopax rusticola
- American Woodcock, Scolopax minor
- Amami Woodcock, Scolopax mira
- Javan Woodcock, Scolopax saturata
- New Guinea Woodcock, Scolopax rosenbergii
- Bukidnon Woodcock, Scolopax bukidnonensis
- Sulawesi Woodcock, Scolopax celebensis
- Moluccan Woodcock, Scolopax rochussenii
- Imperial Snipe, Homoscolopax imperialis
- Giant Snipe, Xylocota undulata
- Jameson's Snipe, Chubbia jamesoni
- Fuegian Snipe, Chubbia stricklandii
- †North Island Snipe, Coenocorypha barrierensis
- Subantarctic Snipe, Coenocorypha aucklandica
- Chatham Snipe, Coenocorypha pusilla
- †South Island Snipe, Coenocorypha iredalei
- Snares Snipe, Coenocorypha huegeli
- Great Snipe, Telmatias medius
- Wood Snipe, Telmatias nemoricola
- Solitary Snipe, Telmatias solitarius
- Latham's Snipe, Telmatias hardwickii
- Pin-tailed Snipe, Telmatias stenurus
- Swinhoe's Snipe, Telmatias megalus
- Noble Snipe, Gallinago nobilis
- Puna Snipe, Gallinago andina
- Pantanal Snipe, Gallinago paraguaiae
- Magellanic Snipe, Gallinago magellanica
- African Snipe, Gallinago nigripennis
- Madagascan Snipe, Gallinago macrodactyla
- Common Snipe, Gallinago gallinago
- Wilson's Snipe, Gallinago delicata
Tringinae: Phalaropes and Shanks Rafinesque, 1815
- Terek Sandpiper, Xenus cinereus
- Common Sandpiper, Actitis hypoleucos
- Spotted Sandpiper, Actitis macularius
- Wilson's Phalarope, Steganopus tricolor
- Red-necked Phalarope, Phalaropus lobatus
- Red Phalarope / Gray Phalarope, Phalaropus fulicarius
- Green Sandpiper, Tringa ochropus
- Solitary Sandpiper, Tringa solitaria
- Gray-tailed Tattler, Heteroscelus brevipes
- Wandering Tattler, Heteroscelus incanus
- Marsh Sandpiper, Totanus stagnatilis
- Wood Sandpiper, Totanus glareola
- Common Redshank, Totanus totanus
- Lesser Yellowlegs, Totanus flavipes
- Spotted Redshank, Totanus erythropus
- Common Greenshank, Totanus nebularia
- Greater Yellowlegs, Totanus melanoleuca
- Nordmann's Greenshank, Totanus guttifer
- Western Willet, Totanus inornata
- Eastern Willet, Totanus semipalmata
Arenariinae: Turnstone and Stints Stejneger, 1885 (1840)
- †Kiritimati Sandpiper, Prosobonia cancellata
- Tuamotu Sandpiper, Prosobonia parvirostris
- †Tahiti Sandpiper, Prosobonia leucoptera
- †Moorea Sandpiper, Prosobonia ellisi
- Ruddy Turnstone, Arenaria interpres
- Black Turnstone, Arenaria melanocephala
- Great Knot, Calidris tenuirostris
- Red Knot, Calidris canutus
- Surfbird, Calidris virgata
- Ruff, Philomachus pugnax
- Broad-billed Sandpiper, Limicola falcinellus
- Sharp-tailed Sandpiper, Limicola acuminata
- Curlew Sandpiper, Erolia ferruginea
- Stilt Sandpiper, Micropalama himantopus
- Temminck's Stint, Eurynorhynchus temminckii
- Long-toed Stint, Eurynorhynchus subminuta
- Red-necked Stint, Eurynorhynchus ruficollis
- Spoon-billed Sandpiper, Eurynorhynchus pygmeus
- Dunlin, Pelidna alpina
- Purple Sandpiper, Pelidna maritima
- Rock Sandpiper, Pelidna ptilocnemis
- Buff-breasted Sandpiper, Ereunetes subruficollis
- Sanderling, Ereunetes albus
- Little Stint, Ereunetes minutus
- Baird's Sandpiper, Ereunetes bairdii
- Least Sandpiper, Ereunetes minutillus
- White-rumped Sandpiper, Ereunetes fuscicollis
- Pectoral Sandpiper, Ereunetes melanotos
- Semipalmated Sandpiper, Ereunetes pusillus
- Western Sandpiper, Ereunetes mauri
Turnici Huxley, 1868
The Turnici are sister to the Limicoli. Using a blend of Černý and Natale (2022) and Stiller et al. (2024) dates the split between the Limicoli and Turnici at about 53 mya. We divide the Turnici into two parvorders: Turnicida and Larida. This split dates to about 49 mya.
Turnicida Huxley, 1868
The buttonquail family (Turnicidae) is the only family in Turnicida. For a long time, the buttonquail weren't considered to be related to shorebirds. They were often grouped with the plains-wanderer and put in the Gruiformes. Of course, both are now in Charadriiformes, but in different places. Although the Monroe and Sibley (1993) checklist removed them from the Gruiformes, they were placed in their own parvclass, with no close relatives.
In their pioneering study of the Charadriiformes, Paton et al. (2003) attempted to use Turnix as an outgroup. After all, van Tuinen et al. (2000) had Turnix outside the shorebirds, but close enough to make a useful outgroup. They got a surprise! They found the buttonquail were well-embedded in the Charadriiformes, sister to the group I now call Lari. And as other studies have also found, the division between the Turnicida and Larida appears deep. That's why I maintain them as distinct parvorders.
Turnicidae: Buttonquail G.R. Gray, 1840 (1831)
2 genera, 18 species HBW-3
 |
| Click for Turnicidae genus tree |
|---|
The buttonquail tree was constructed by combining the species groups described by Debus (1996) with the 7-species phylogeny of Černý and Natale (2022). I was surprised there were no conflicts. The fact that there weren't reminds us that traditional methods often did a pretty good job of determining the phylogeny. Debus also describes the morphological differences between quail and buttonquail that justify grouping them together, including Ortyxelos. See also the discussion in Sibley and Ahlquist (1990).
I do have a reservation about the resulting phylogeny. It seems rather odd to have two African species (Hottentot and Black-rumped Buttonquail) embedded in an Australasian clade.
The extinct New Caledonian Buttonquail, Turnix novaecaledoniae, is split from an Australian species, Painted Buttonquail, Turnix varius, based on morphological differences and its remote former range (HBW/BirdLife).
Species List: Turnicidae (Buttonquail)
- Quail-plover, Ortyxelos meiffrenii
- Yellow-legged Buttonquail, Turnix tanki
- Spotted Buttonquail, Turnix ocellatus
- Barred Buttonquail, Turnix suscitator
- Madagascan Buttonquail, Turnix nigricollis
- Common Buttonquail, Turnix sylvaticus
- Red-backed Buttonquail, Turnix maculosus
- Little Buttonquail, Turnix velox
- Worcester's Buttonquail, Turnix worcesteri
- Sumba Buttonquail, Turnix everetti
- Red-chested Buttonquail, Turnix pyrrhothorax
- Black-rumped Buttonquail, Turnix nanus
- Hottentot Buttonquail, Turnix hottentottus
- Black-breasted Buttonquail, Turnix melanogaster
- Painted Buttonquail, Turnix varius
- Chestnut-backed Buttonquail, Turnix castanotus
- Buff-breasted Buttonquail, Turnix olivii
- †New Caledonian Buttonquail, Turnix novaecaledoniae
Larida Sharpe 1891
Larida includes more than just the gulls and terns. Larida contains three superfamilies, Glareoloidea (crab plover, coursers, pratincoles), and the two sister superfamilies Alcoidea (skuas and auks), and Laroidea (skimmers, terns and noddies, and gulls).
Černý and Natale (2022) and Stiller et al. (2024) give different dates for the split between the Glareoloidea and the combined Alcoidea and Laroidea. I'm using a compromise date of 38 mya for that split and 35.5 mya for the Alcoidea/Laroidea. This justifies superfamily status for all three groups. The dates near Alcidae are probably most accurate as all six of Černý and Natale's Larida calibration points lie there. Some of the Stiller et al. dates proved to be too young as they contradicted fossil evidence used by Černý and Natale. Because of this, I've generally given preference to Černý and Natale's date for Larida due to much better calibration. This is discussed further in connection with the alcids.
Glareoloidea Sharpe, 1891
 |
| Click for Crab Plover, Courser, and Pratincole tree |
|---|
A group similar to TiF's Glareoloidea has been known since the 19th century, usually as Glareolidae, but sometimes as Cursoriidae, as in Sharpe (1896). Sharpe included the pratincoles and coursers in this group, but he also included the Crab Plover, Dromas ardeola, Quail-plover, Ortyxelos meiffrenii, and the Egyptian Plover, Pluvianus aegyptius. Since then, the last species were removed from the Glareolidae, leaving just the pratincoles and coursers. The Crab Plover was not removed very far, and has its own family in the superfamily Glareoloidea.
The Quail-plover, Ortyxelos meiffrenii and Egyptian Plover, Pluvianus aegyptius were eventually found to belong elsewhere. The Quail-plover is in the buttonquail family, Turnicidae. The most recent common ancestor is currently thought to have lived about 49 mya. The Egyptian Plover was removed even further. It is now placed in its own parvclass (!), Pluvianida. The most recent common ancestor is the common ancestor of all extant Charadriiformes, living perhaps 58 mya.
TiF continues this trend of putting the members of Sharpes' Glareolidae by raising the subfamilies Glareolinae and Cursoriinae to family rank.
Splitting the Glareolidae
While Stiller et al. (2024) date the split between Glareolinae and Cursoriinae at about 23 mya, Černý and Natale put it at 33.5 mya! The Stiller ages need to be increased due to the change in alcid ages. My current guess for the age of the split between pratincoles and coursers is 27 mya. That gave me a bit of guidance for reassigning genera. It also opens the question of whether the pratincoles and coursers should be considered separate families.
The separation into two families clearly passes the age test, even using the Stiller et al. age of 23 mya. It's also that the pranticoles and coursers are distinct groups. That combination justifies treating each group at family rank — Glareolidae and Cursoriidae.
Dromadidae: Crab Plover G.R. Gray, 1840
1 genus, 1 species HBW-3
The Crab Plover's nearest relatives are the coursers and pratincoles, the Glareolidae and Cursoriidae. I'm giving the split between the families a compromise date of 33 million years ago.
Using DNA hybridization, Sibley and Ahlquist (1990) found that Dromas is sister to the Glareolidae and Cursoriidae. This was confirmed by Pereira and Baker (2010), who used the more accurate DNA sequencing on data from pratincoles, coursers, and a number of other shorebird taxa. These results are supported by the comprehensive analysis of Charadriiformes by Černý and Natale (2022).
- Crab Plover, Dromas ardeola
Glareolidae: Pratincoles C.L. Brehm, 1831
4 genera, 8 species HBW-3
The odd name praticole means meadow-dweller. It's based on the latin words pratum (meadow) and incola (inhabitant). The arrangement of the pratincoles and coursers is largely based on the genetic analyses of Cohen (2011), which uses up to four genes (ND2, Fib5, TGFB, GAPDH), and that of Černý and Natale (2022), who sampled half of the Glareolidae and Cursoriidae.
More specifically, the tree I use is based on Supplemental Figure A.33 in Černý and Natale (2022), (A.31 differs slightly), and with Figures 4.1 (total evidence) and 4.3 (nuclear + mitochondrial DNA) from Cohen (2011).
I now use 4 genera for the pratincoles, instead of the more usual 1 or 2. There are no calibration points anywhere in Glareolidae or Cursoriidae. Moreover, the total evidence tree in Černý and Natale (2022) is somewhat different from the phylogeny I am using. This means I have only limited information about ages for deciding genus boundaries in both Glareolidae and Cursoriidae.
Genus boundaries have been changed as follows:
- The Gray Pratincole, Glareola cinerea, and Small Pratincole, Glareola lactea, have been transferred to Galachrysia (Bonaparte 1856, type lactea).
- The Rock Pratincole, Glareola nuchalis, and Madagascan Pratincole, Glareola ocularis, have been transferred to Subglareola (Mathews 1913, type ocularis).
- I have restored the monotypic genus Stiltia (G.R. Gray, 1855, type isabella) which I had previously submerged in Glareola.
Species List: Glareolidae (Pratincoles)
- Rock Pratincole, Subglareola nuchalis
- Madagascan Pratincole, Subglareola ocularis
- Australian Pratincole, Stiltia isabella
- Gray Pratincole, Galachrysia cinerea
- Small Pratincole, Galachrysia lactea
- Oriental Pratincole, Glareola maldivarum
- Collared Pratincole, Glareola pratincola
- Black-winged Pratincole, Glareola nordmanni
Cursoriidae: Coursers G.R. Gray, 1840
3 genera, 9 species HBW-3
I had previously used two genera for the coursers, but based on what I can tell from the phylogeny, now think it's better to use three.
- The Double-banded Courser, previously Rhinoptilus africanus, has been transferred to Smutsornis (Roberts 1922, monotypic).
Species List: Cursoriidae (Coursers)
- Double-banded Courser, Smutsornis africanus
- Three-banded Courser, Rhinoptilus cinctus
- Bronze-winged Courser, Rhinoptilus chalcopterus
- Jerdon's Courser, Rhinoptilus bitorquatus
- Cream-colored Courser, Cursorius cursor
- Somali Courser, Cursorius somalensis
- Burchell's Courser, Cursorius rufus
- Temminck's Courser, Cursorius temminckii
- Indian Courser, Cursorius coromandelicus
Alcoidea Leach, 1820
Stiller et al. (2024) placed the split between Alcoidea and the Laroidea split at about 27 million years ago. They were wrong. Even Alcidae is quite a bit older than that! The oldest fossil that is fairly clearly an Alcid is dated at 34.44 mya, which is calibration point 6 in Černý and Natale (2022). See Chandler and Parmley (2003) and the discussion in Smith (2015) and Smith and Clarke (2015). Further information can be found in Smith (2011). Of course, the auk family must be even older.
Černý and Natale date the auk-skua split to about 35.5 mya, and the Alcoidea-Laroidea split a million years earlier. I'm going with those numbers, but they may still be too young. This change in dating compared with Stiller et al. has ripple effects throughout the Turnici.
Stercorariidae: Skuas, Jaegers G.R. Gray, 1870 (1831)
2 genera, 7 species HBW-3
The skuas were formerly separated in the genus Catharacta, but a genetic study by Cohen et al. (1997) found the Great Skua and Pomarine Jaeger are sister species. This has been called into question by Braun and Brumfield (1998), who argued that all the skuas might be sister to the Pomarine Jaeger. At the time, the 2-genus treatment seemed untenable, and most have decided to merge Catharacta into Stercorarius. If Braun and Brumfield are right, an alternative would be to put the Pomarine Jaeger by itself in Coprotheres (Reichenbach 1852) and the skuas in Catharacta.
The results in Černý and Natale (2022) open the door to another option. They found that the Long-tailed and Parasitic Jaegers are sister species. Moreover, they are not closely related to the other skuas. This suggests using two genera, and I do so. I've left those two in Stercorarius, Brisson 1760, type parasiticus and moved the rest into Catharacta, Brunnich 1764, type skua (for most of them it's back into Catharacta).
| Jaeger/Skua tree |
|---|
 |
|
Sources: Blechschmidt et al. (1993), Braun and Brumfield (1998), Černý and Natale (2022), Cohen et al. (1997), Ritz et al. (2008). |
The species boundaries in the southern skuas are somewhat uncertain. See Ritz et al. (2008).
Species List: Stercorariidae (Skuas, Jaegers)
- Long-tailed Jaeger, Stercorarius longicaudus
- Parasitic Jaeger / Arctic Skua, Stercorarius parasiticus
- Pomarine Jaeger / Pomarine Skua, Catharacta pomarina
- Great Skua, Catharacta skua
- Brown Skua, Catharacta antarctica
- Chilean Skua, Catharacta chilensis
- South Polar Skua, Catharacta maccormicki
Alcidae: Auks Leach, 1820
12 genera, 25 species HBW-3
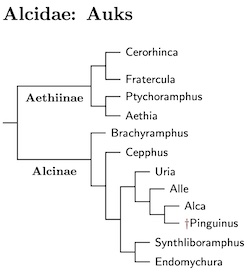 |
| Click for Alcidae species tree |
|---|
Auk taxonomy follows Baker et al. (2007) and Pereira and Baker (2008). Indeed, the current TiF tree, from Černý and Natale (2022) differs from theirs only in the Aethia auklets, which were mostly unresolved by Pereira and Baker.
The time scale in Pereira and Baker should be taken with a grain of salt. Considering that some of the fossils used as calibration points are distantly related, or of controversial affinities, perhaps with a whole truckload of salt would be better. To make that clear, Pereira and Baker (2008), using bad calibrations, put the skua/auk split at about 62 mya, while Stiller et al. (2024) date it around 25 mya. In fairness to Pereira and Baker, their calibrations seemed more reasonable at the time. Sixteen years made a huge difference in what is considered reasonable.
Humphries and Winker (2010) resolved the Aethia auklets, but their solution differs from Černý and Natale, which I follow.
There is some ambiguity in Černý and Natale (2022) concerning the position of the Brachyramphus and Synthliboramphus (including Endomychura) murrelets. See Figure 4 and surrounding material. Both positions have relatively poor support. I've opted to Fig. 4a, which is based on Supplemental Figure A.31.
Xantus's Murrelet Split: For the split of Scripps's Murrelet, Synthliboramphus scrippsi, from Xantus's Murrelet, Synthliboramphus hypoleucus, see Birt et al. (2012). Further, Synthliboramphus hypoleucus takes the name Guadalupe Murrelet.
Tropical Murrelets: Based on the dates from Černý and Natale (2022), I've moved the tropical murrelets to genus Endomychura (Oberholser 1899, type hypoleuca).
Species List: Alcidae (Auks)
Aethiinae AOU 1908 (1840)
- Rhinoceros Auklet, Cerorhinca monocerata
- Tufted Puffin, Fratercula cirrhata
- Atlantic Puffin, Fratercula arctica
- Horned Puffin, Fratercula corniculata
- Cassin's Auklet, Ptychoramphus aleuticus
- Crested Auklet, Aethia cristatella
- Whiskered Auklet, Aethia pygmaea
- Parakeet Auklet, Aethia psittacula
- Least Auklet, Aethia pusilla
Alcinae Leach 1820
- Long-billed Murrelet, Brachyramphus perdix
- Marbled Murrelet, Brachyramphus marmoratus
- Kittlitz's Murrelet, Brachyramphus brevirostris
- Black Guillemot, Cepphus grylle
- Spectacled Guillemot, Cepphus carbo
- Pigeon Guillemot, Cepphus columba
- Razorbill, Alca torda
- †Great Auk, Pinguinus impennis
- Dovekie / Little Auk, Alle alle
- Thick-billed Murre / Brunnich's Guillemot, Uria lomvia
- Common Murre / Guillemot, Uria aalge
- Ancient Murrelet, Synthliboramphus antiquus
- Japanese Murrelet, Synthliboramphus wumizusume
- Craveri's Murrelet, Endomychura craveri
- Scripps's Murrelet, Endomychura scrippsi
- Guadalupe Murrelet, Endomychura hypoleuca
Laroidea Rafinesque, 1815
Gulls, Skimmers, Terns, and more: Five Clades
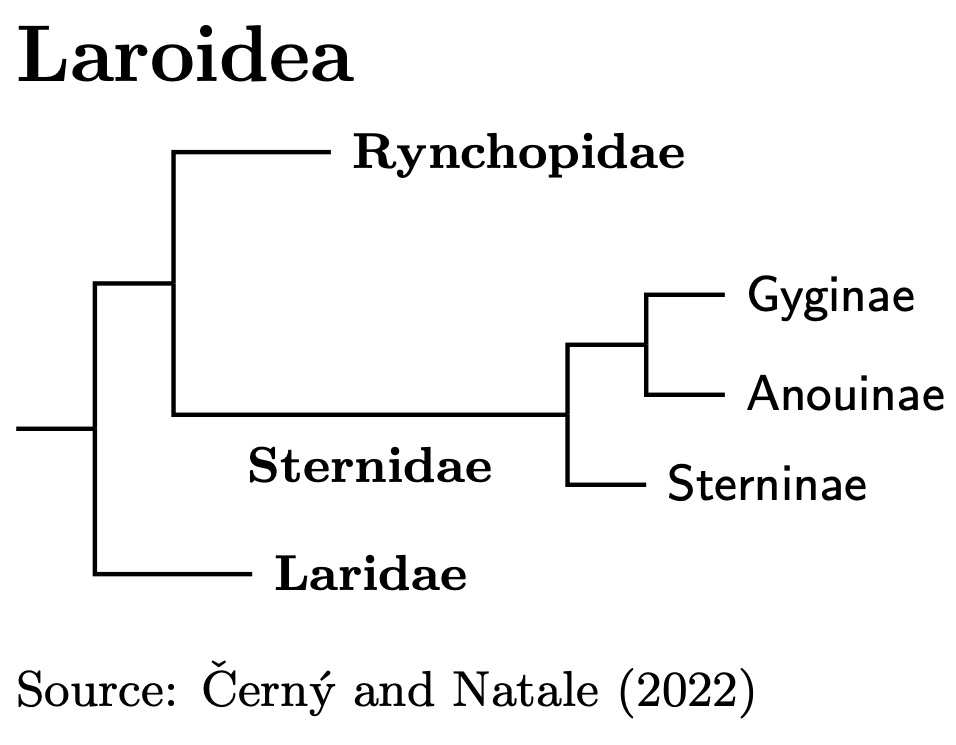 |
TiF follows Černý and Natale's (2022) branching order for Laroidea. The first split is between the gull family Laridae, and everything else. Since there are more gull species, I put Laridae first.
The other branch contains two families, the skimmers (Rynchopidae) and the tern family Sternidae. Sternidae is divided into three subfamilies. The first, Gyginae contains the fairy terns, a.k.a. white terns or white noddies of genus (Gygis) it is sister to Anouinae, the noddies. This pair of small subfamilies is sister to the rest of the terns, Sterninae.
Including the gulls, we have 5 deep branches. Since the family Laridae is often considered to contain all 5 groups (but not by me), I'll temporarily rank them all as subfamilies in order to explain why I divide them into three families. The subfamilies are — Rynchopinae (skimmers), Gyginae (fairy-terns), Anouinae (noddies), Sterninae (terns), and Larinae (gulls).
Arranging the Five Subfamilies
Before proceeding, there are two credible alternatives that we need to consider: Dufour et al. (2024, Fig. S1) and SACC proposal #1032. Figure 44 in the proposal, taken from Thibault and Cibois (2017) appears quite different from the TiF tree. Although Figure 44 cites several papers, only Baker et al. (2007) is relevant for arranging the subfamilies.
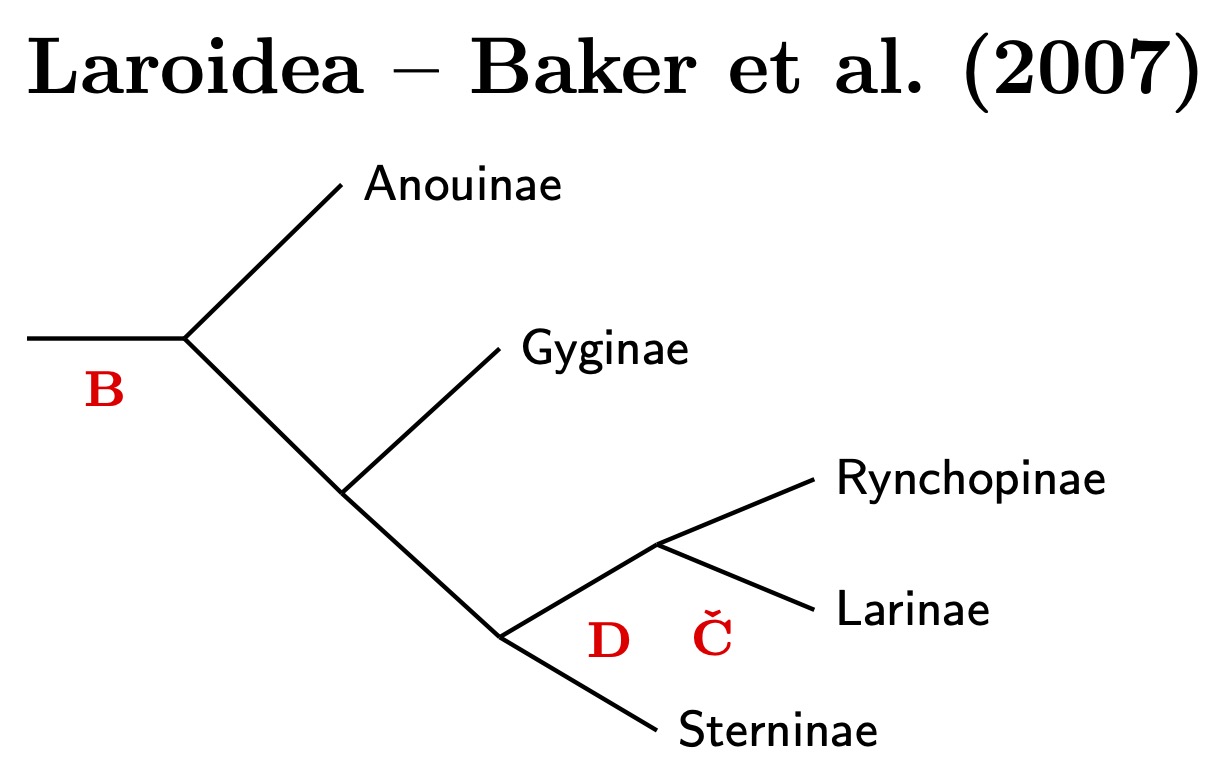 |
| Click diagram for the 3 arrangements |
|---|
The Laroida diagram shows the subfamilies according to Baker et al. (2007). The red B denotes the root according to Baker et al. The red Č shows the root from Černý and Natale (2022). Finally, the red D marks the root as in Dufour et al.
To see it works, we start with the Baker tree, although it may be easiest to see this from the unrooted tree. Notice that the red is at the actual root. To get the Černý and Natale tree, we start at the branch labelled Č, the Larinae branch. There are two ways we can go from Č. We can go to Larinae, the basal branch in Černý and Natale's tree, or we can go toward the other Laroidea.
Proceeding along, we reach a second fork. It can be taken to Rynchopinae, or to the remaining three subfamilies. That means that Rynchopinae is the second branch in Černý and Natale's tree. Continuing past D to the next fork, we have two options: continue to Sterninae or to Anouinae + Gyginae. So the next branch of the Černý and Natale tree is Sterninae, with the next one leading to the final fork, between Anouinae and Gyginae. That gives us the Černý and Natale tree!
If we'd started at D, we would have obtain the Dufour et al. tree instead. This method works equally well no matter which of the possible roots we use, even the unmarked ones. This procedure only gives us equivalent trees (ignoring the change of root). It can't yield a fundamentally different tree, such as one where Gyginae and Larinae are sister taxa.
The tree shown in Figure 44 of the SACC proposal, and the Černý and Natale (TiF) tree are actually quite similar. The only difference between them is the position of the root. Is it at point B on the far left side? Or is it at point Č?
So which root is the right one? I don't know for certain. Checking the support values suggest the Černý and Natale (2022) topology is somewhat soft, but Baker et al. (2007) looks pretty good. But there is another consideration. Although misrooting is rare now, it wasn't always. Indeed, it was fairly common as recently as fifteen years ago, and sometimes also applied to subtrees. One important factor when choosing between the two trees is that Černý and Natale use more data. They consider more DNA per taxon, and, importantly, include a lot more taxa. My earlier experience with rooting problems was that both types of additional tend to yield better results. It is why I bet on Černý and Natale over Baker et al.
There's one more relevant paper that uses even more data, but on fewer taxa — Stiller et al. (2024). Stiller et al. threw a large amount of data at the problem of bird phylogeny. The Laroidea they included in their analysis were members of Rynchopinae, Sterninae, and Larinae. They found Rynchopinae closer to Sterninae than to Larinae. This is consistent with the TiF tree (now based on Černý and Natale), but not with either Baker et al., which was used by Thibault and Cibois (2017) and in SACC proposal #1032, or with Dufour et al. (2024, Fig. S1).
Aging the Five Subfamilies
Now that we've settled on an arrangement of the subfamilies, we can inquire about their age. Just how old are these subfamilies? Stiller et al. (2024) estimated the split between the gulls and terns occurred about 20 mya, and the Rynchops branch at about 19 mya. The Anous-Gygis clade was not included there, but Černý and Natale found it was only slightly younger, perhaps by 0.3 million years.
But that can't be right! The old alcid fossil that Černý and Natale (2022) used forces the Larids to be older than Stiller et al. (2024) estimates. Černý and Natale have already taken this into account with their calibrations, so we use their dates. That means the split between Larinae and the four subfamilies dates to 27.5 mya, and that between Rynchopinae and the Sterninae group to 26 mya. Going further, the Sterninae split from Anouinae and Gyginae maybe a half million years later (25.5 mya), with the Anouinae/Gyginae split at 24.5 mya.
Given the ages, we have to consider all 5 subfamilies as potential families. There are substantial morphological and vocal differences between gulls and terns, I'm happy treating them as families. The skimmers are very distictive and deserve their own family. There are no other birds like them. The Anouinae, Gyginae, and Sterninae seem much more similar, although there are some differences between noddies and terns. Because the similarities are stronger, I've put them all in Sternidae. The Gyginae and Anouinae are sister taxa, and together are basal in Sternidae.
Rynchopidae: Skimmers Bonaparte, 1838
1 genus, 3 species HBW-3
 |
| Rynchopidae species tree |
|---|
The skimmer family Rynchopidae is the sister group of the Sternidae (fairy-terns, noddies and terns). Their common ancestor lived an estimated 26 mya (Černý and Natale, 2022). It is quite a distinctive bird. As Alvaro Jaramillo wrote concerning SACC proposal #810: “This group is one of the most unusual on earth based on its bill shape.” Not only is the upper bill is much shorter than the lower, but both are extremely laterally compressed. From the front, it's like looking at a knife-blade. Presumably these are adaptations that help it fish by skimming along just above the water, with the lower beak actually in the water. The narrowness of the bill reduces water resistence, and the short upper mandible usually stays out of the water. Another unusual trait of the skimmers is that they often rest on the shore in a posture that makes them appear dead.
Species List: Rynchopidae (Skimmers)
- African Skimmer, Rynchops flavirostris
- Indian Skimmer, Rynchops albicollis
- Black Skimmer, Rynchops niger
Sternidae: Fairy-Terns, Noddies, Terns Vigors, 1825
11 genera, 49 species HBW-3
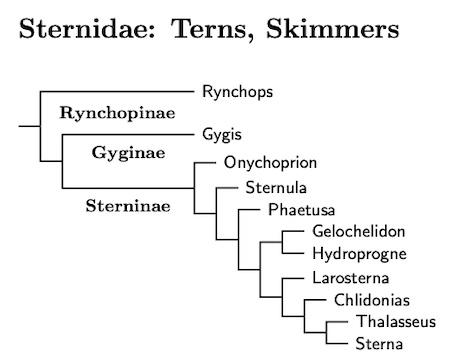 |
| Click for Sternidae species tree |
|---|
There are four older relevant papers on tern phylogeny: Bridge et al. (2005), Baker et al. (2007), Ödeen et al. (2010), and Jackson et al. (2012). They successively analyzed more genes, but Baker et al. has better taxon sampling in the terns. From those papers, it was already clear that the skimmers are by themselves, the gulls group together, and the terns other than Anous and Gygis also form a deep clade. These earlier papers were unable to conclusively show if all of the terns are monophyletic, or whether Anous and Gygis needed to be removed to make them monophyletic.
For the terns and allies, both Černý and Natale (2022) and Dufour et al. (2024, Fig. S1) agree on the subfamily structure. Gygis and Anous are long-separated sister taxa, and together are sister to the rest of the terns.
Gyginae: Fairy-Terns Verheyen, 1959
The fairy terns or white terns of genus Gygis are only distantly related to Anous. In fact, they are so distantly related that we have put them in their own subfamily.
Based on SACC Proposal #1032 and the comments and references therein, including Olson (1994), and Pratt (2020), I've split the White Tern, Gygis alba, into Atlantic White-Tern, Gygis alba and Common White-Tern, Gygis candida. Note that Olson (1994) is embedded in the SACC proposal, including notes from Claudia Wilds.
Fairy Tern or White Tern?
But there is one charming bird: it is a small, snow-white tern, which smoothly hovers at the distance of a few feet above one's head, its large black eye scanning, with quiet curiosity, your expression. Little imagination is required to fancy that so light and delicate a body must be tenanted by some wandering fairy spirit. -- Charles Darwin, Voyage of the Beagle, 1845 ed., Chap. XX.
The OED tells us that the earliest known use of Fairy Tern is by Thomas Rymer Jones. He translated the Birds section of Alfred Brehm's Brehms Tierleben (Brehm's Life of Animals) in 4 volumes from 1869-1873. Brehm was the son of the ornithologist Christian Ludwig Brehm. The name Fairy Tern appears in vol. 4. It is not used in the species account on pg. 183. Rather, it only appears in the Classification of Birds, on page 296, where it says “The Fairy Terns (Gygis), iv., 183; the White or Silky Tern (G. candida)”. That said, the species account cites Darwin's description of the bird, which uses the term “fairy”. That seems to be the ultimate origin of the name Fairy Tern. Darwin saw them in the Cocos (Keeling) Islands, so they were probably G. candida, not G. microrhyncha.
I've adopted Fairy Tern rather than White Tern in the primary name for the three Gygis species. This name, which was originally applied to Gygis, has been in common use among the public for many years. In Hawaii, G. candida is often called the White Fairy Tern. Using the tern Fairy-Tern helps emphasize that the Gyginae are only distantly related to the main body of the terns. Their most recent common ancestor is believed to have lived perhaps 18.5 millon years ago. It's written as Fairy-Tern, with the hypen indicating a group name. Please don't confuse it with the Australian Fairy Tern Sternula nereis. For the present, I continue to include White-Tern as a secondary name for all three Gygis species.
- Atlantic Fairy-Tern / Atlantic White-Tern, Gygis alba
- Common Fairy-Tern / Common White-Tern, Gygis candida
- Little Fairy-Tern / Little White-Tern, Gygis microrhyncha
Anoinae: Noddies Bonaparte 1854
The Noddy portion of the tree agrees with Cibois et al. (2016), who found that Procelsterna is embedded in Anous. There is an alternate, which is to split them into three genera: Anous, Megalopterus, and Procelsterna.
According to Cibois et al. the noddy clade is of recent origin, in which case it makes sense to treat them all as a single genus. Anous (Stephens 1826, type stolidus) has priority over Procelsterna (Lafresnaye 1842, type ceruleus teretriostris). That is what I do here. However, if you believe the clade is substantially older, as in Černý and Natale (2022), the three genus solution would be preferrable.
- Brown Noddy, Anous stolidus
- Black Noddy, Anous minutus
- Lesser Noddy, Anous tenuirostris
- Blue-gray Noddy / Blue Noddy, Anous ceruleus
- Gray Noddy, Anous albivitta
Sterninae: Terns Vigors, 1825
There are differences in the tern taxonomy in Bridge et al. (2005) and Baker et al. (2007). I've adopted the Bridge et al. framework because they sampled many more tern species than Baker et al. Use of Černý and Natale (2022) hasn't changed this much.
However, Černý and Natale did turn up and interesting possibility in genus Sterna. It's not clear that Forster's Tern, Sterna forsteri, and the Snowy-crowned Tern, Sterna trudeaui, belong in Sterna. Černý and Natale's genetic phylogenies have the sister species forsteri and trudeaui as sisters to a Thalasseus plus the remaining Sterna.
That said, the branch length between the three is very short, and the support for this is weak. There's 100% posterior support for Sterna plus Thalasseus, for Thalasseus alone, for the fosteri pair, and for the remaining Sterna (i.e., minus the fosteri pair). However, their preferred phylogeny has only 48% support for Thalasseus plus the remaining Sterna. Presumably, other arrangements of the three clades were even less supported. Still, other papers such as Bridge et al. (2005) and Dufour et al. (2024, Fig. S1) have found no such division of Sterna although the branch length was short. For the present, I retain an undivided Sterna.
English Name Change
Because of the possible confusion between the Gygis Fairy-Terns and the Fairy Tern, Sternula nereis, I've switched to the Clements name Australian Fairy Tern for Sternula nereis.
Sterninae: Tern Splits
Australian Tern: The Australian Tern, Gelochelidon macrotarsa has been split from Gull-billed Tern, Gelochelidon nilotica, based on morphological and life history differences. What little DNA evidence is available could be construed either to support or oppose the split.
Cabot's Tern: The American Sandwich Terns are split under the old name Cabot's Tern, Thalasseus acuflavidus. Efe et al. (2009) found that Cabot's Tern is more closely related to Elegant Tern than to Old World Sandwich Terns. They also found that no systematic genetic distinction between Cayenne and Cabot's Terns and question whether eurygnathus is distinct from acuflavidus.
West African Crested Tern: The West African Crested Tern, Thalasseus albididorsalis, has been split from the Royal Tern, Thalasseus maximus. DNA (Collinson et al., 2017) shows it is sister to the Lesser Crested Tern, Thalasseus bengalensis.
The last two terns on the list lack genetic data, but seem to belong in Sterna.
Sterninae: Terns Vigors, 1825
- Aleutian Tern, Onychoprion aleuticus
- Sooty Tern, Onychoprion fuscatus
- Bridled Tern, Onychoprion anaethetus
- Gray-backed Tern / Spectacled Tern, Onychoprion lunatus
- Damara Tern, Sternula balaenarum
- Little Tern, Sternula albifrons
- Australian Fairy Tern, Sternula nereis
- Saunders's Tern, Sternula saundersi
- Least Tern, Sternula antillarum
- Yellow-billed Tern, Sternula superciliaris
- Peruvian Tern, Sternula lorata
- Large-billed Tern, Phaetusa simplex
- Caspian Tern, Hydroprogne caspia
- Gull-billed Tern, Gelochelidon nilotica
- Australian Tern, Gelochelidon macrotarsa
- Inca Tern, Larosterna inca
- Whiskered Tern, Chlidonias hybrida
- Black-fronted Tern, Chlidonias albostriatus
- Black Tern, Chlidonias niger
- White-winged Tern, Chlidonias leucopterus
- Elegant Tern, Thalasseus elegans
- Cabot's Tern, Thalasseus acuflavidus
- Sandwich Tern, Thalasseus sandvicensis
- Great Crested Tern / Greater Crested Tern, Thalasseus bergii
- Chinese Crested Tern, Thalasseus bernsteini
- Royal Tern, Thalasseus maximus
- West African Crested Tern, Thalasseus albididorsalis
- Lesser Crested Tern, Thalasseus bengalensis
- Forster's Tern, Sterna forsteri
- Snowy-crowned Tern, Sterna trudeaui
- Arctic Tern, Sterna paradisaea
- South American Tern, Sterna hirundinacea
- Kerguelen Tern, Sterna virgata
- Antarctic Tern, Sterna vittata
- Common Tern, Sterna hirundo
- White-cheeked Tern, Sterna repressa
- Black-naped Tern, Sterna sumatrana
- Roseate Tern, Sterna dougallii
- White-fronted Tern, Sterna striata
- River Tern, Sterna aurantia
- Black-bellied Tern, Sterna acuticauda
Laridae: Gulls Rafinesque, 1815
13 genera, 57 species HBW-3
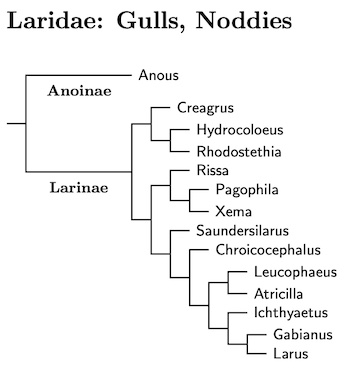 |
| Click for Laridae species tree |
|---|
At the generic level, the present TiF taxonomy of the gulls is a refinement of Sternkopf (2011) and Pons et al. (2005). Sternkopf used a wider variety of genes than Pons et al., but cut back a bit on taxa considered. Their results are generally consistent with each other and with Crochet et al., (2000). The Pons et al. treatment was quickly accepted by AOU and BOU. The recent paper by Černý and Natale (2022) increased certainty about some of the nodes, and broke ties in a couple of cases.
The use of Sternkopf (2011) and Černý and Natale (2022) means that the Little/Ross's Gull clade is one of the basal groups and that the Ring-billed and Common Gulls are sister species. It's also led to some minor reordering within genera. Although Sternkopf did not consider Saunder's Gull, Černý and Natale (2022) did, its current position on a branch just before Chroicocephalus reflects that.
As recently as H&M-3, most gulls were put in genus Larus. The exceptions were pretty clearly different: Swallow-tailed Gull (Creagrus), Ross's Gull (Rhodostethia), Ivory Gull (Pagophila), Sabine's Gull (Xema), the two kittiwakes (Rissa), and the Dolphin Gull (Leucophaeus). Everything else was in Larus.
This classification started to change once the DNA was studied. Pons et al. (2005) started the changes with by noting the various types of gulls on their consensus tree (Fig. 2). Besides those listed above (except Dolphin Gull), they separated Saunder's Gull, the masked gulls, hooded gulls, black-headed gulls, band-tailed gulls, and white-headed gulls. Pretty soon, Saunder's Gull moved to genus Saundersilarus, the masked gulls to Chroicocephalus, hooded gulls and Dolphin Gull to Leucophaeus, black-headed to Ichthyaetus, and band-tailed and white-headed gulls remained in Larus, as in H&M-4.
New and Not so New Genera: Consideration of Černý and Natale (2021, 2022) allowed some refinement of this arrangement.
- The Slender-billed Gull, Chroicocephalus genei, is distinctive when compared to the other Chroicocephalus gulls. So even so the separation between it and Chroicocephalus only occurred about 4 million years ago (Černý and Natale, 2022), I've placed it to genus Gelastes (Bonaparte 1856, monotypic). (2021)
- The division between Dolphin and Gray Gulls on one hand, and Laughing, Franklin's, and Lava Gulls on the other is about 6 million years, enough to support different genera. Moreover, they obviously form two, or even three groups. Accordingly, the Laughing, Franklin's and Lava Gulls have been moved to Atricilla (Bonaparte 1854, type atricilla). It would not be unreasonable to also split the Gray Gull, Leucophaeus modestus. In that case it would be genus Blasipus (Bruch 1853). (2021)
- The band-tailed gulls (4 species) have been moved to genus Gabianus (Bruch 1853, type pacificus) on the grounds that they are distinctive, even though the temporal separation from the Larus gulls is only about 3 million years (Černý and Natale, 2022).
When it comes to Larus, we have more information, and we need all we can get! The papers by Liebers et al. (2001, 2002, 2004), de Knijff et al. (2001), Crochet et al. (2002), Pons et al. (2004), and Gay et al. (2005) focused on the big white-headed gulls. They also get full coverage by Sternkopf (2011) and Černý and Natale (2021, 2022).
In spite of all the above effort, Larus gull relationships remain somewhat obscured by past and recent hybridization. Questions about possible ring species have been raised, and refuted (Liebers et al., 2004). Species limits within the herring gulls remain controversial. Nonetheless, a coherent picture is gradually being teased out.
The TiF list has adopted Figs. A-5 and A-6 from Černý and Natale (2021) for the Larus gulls. The published version of Černý and Natale (i.e., 2022) has a lot in common with the preprint, but adds two more versions of Larus gull phylogeny. I don't them as improvements over the previous TiF phylogeny and have left it as it is.
Once we've removed the band-tailed gulls from genus Larus, Heerman's Gull, Larus heermanni is the first branch of the Larus tree. You'll notice it has a full black tail. This differs from both the band-tailed gulls — the nearest relatives of Larus — and the remaining Larus gulls. The second branch is a small clade consisting of smaller white-headed gulls: Ring-billed (delawarensis), Common (canus), and Short-tailed (brachyrhynchus) Gulls. Then we get to the large white-headed gulls.
The big problem in sorting out the phylogeny of the large white-headed gulls is that their 18 species only recently differentiated, perhaps in the last 1.5 million years. In such a case, income lineage sorting is bound to be a problem. The fact that some of these are still hybridizing demonstrates that. Some of the hybrids even have names, such as the Olympic Gull (Glaucous-winged ✗ Western Gull) that is common on the US West Coast, particularly in the Pacific Northwest. Then there's the confusing swarm of different forms of the Iceland Gull (glaucoides, kumlieni, thayeri), which was previously considered to be 2 species. It's a real mess!
There's considerable support for the Yellow-footed Gull, Larus livens, and Western Gull, Larus occidentalis (both races) being likely sisters, and being the sister group to the main group of Larus gulls. Some of the Larus frequently hybridize, even when far apart on the tree. A prime example is the Western Gull, Larus occidentalis, which hybridizes freely with the Glaucous-winged Gull, Larus glaucescens. We shouldn't be that surprised. Far apart on the tree translates to a common ancestor within the last million or so years.
The Kelp Gull, Larus dominicanus, is next, in a group by itself. The remaining Larus gulls form two groups, one contains the European Herring Gull, Larus argentatus, the Lesser Black-backed Gull, Larus fuscus, and some close relatives. The other includes the Great Black-backed Gull, Larus marinus, the American Herring Gull, Larus smithsonianus, Iceland Gull, Larus glaucoides, and the far eastern herring-type gulls.
Laridae — Further Taxonomic Notes
Chroicocephalus: I'm now using Supplementary Figures A.31 and A.33 of Černý and Natale (2022) for Chroicocephalus.
Gray-hooded/headed Gull: The Gray-hooded Gull / Gray-headed Gull, Chroicocephalus cirrocephalus, has been split into the Gray-hooded Gull, Chroicocephalus cirrocephalus, of South America and the Gray-headed Gull, Chroicocephalus poiocephalus, of Africa. Given et al. (2005) found that C. poiocephalus was more closely related to Hartlaub's Gull, Chroicocephalus hartlaubii than to C. cirrocephalus. They noted this relationship could be an artifact of introgression, but they did not have any evidence to that effect.
White-eyed Gull: The exact position of the White-eyed Gull, Ichthyaetus leucophthalmus, remains an issue. Černý and Natale (2022) analyzed two different ways and put it in slightly different locations in Ichthyaetus. There's no doubt its in the black-headed gull clade, but exactly where it goes is rather uncertain.
Thayer's Gull Lump: Some of the species limits among the herring gulls remain contentious. E.g., see Pittaway (1999) and Weir et al. (2000) concerning whether glaucoides, kumlieni, and thayeri are one, two, or even three species. For the present, I am following AOU on this (one). So Thayer's Gull, Larus thayeri, is lumped into Iceland Gull, Larus glaucoides.
Possible Splits: Other named taxa in Liebers's clade II sometimes considered species include (West) Siberian Gull (heuglini and maybe taimyrensis), Baltic Gull (fuscus), Steppe Gull (barabensis). They also suggest there are two types of Glaucous Gull which may not match traditional subspecies, and two types of European Herring Gulls that definitely don't match traditional subspecies. There's also a question concerning whether the Yellow-legged Gull should be split into Mediterranean Yellow-legged Gull (michahellis), Atlantic Yellow-legged Gull (atlantis) and possibly even Cantabrican Yellow-legged Gull (lusitanius?).
- Swallow-tailed Gull, Creagrus furcatus
- Little Gull, Hydrocoloeus minutus
- Ross's Gull, Rhodostethia rosea
- Ivory Gull, Pagophila eburnea
- Sabine's Gull, Xema sabini
- Black-legged Kittiwake, Rissa tridactyla
- Red-legged Kittiwake, Rissa brevirostris
- Saunders's Gull, Saundersilarus saundersi
- Slender-billed Gull, Chroicocephalus genei
- Bonaparte's Gull, Chroicocephalus philadelphia
- Black-headed Gull, Chroicocephalus ridibundus
- Brown-headed Gull, Chroicocephalus brunnicephalus
- Gray-hooded Gull, Chroicocephalus cirrocephalus
- Gray-headed Gull, Chroicocephalus poiocephalus
- Hartlaub's Gull, Chroicocephalus hartlaubii
- Red-billed Gull, Chroicocephalus scopulinus
- Silver Gull, Chroicocephalus novaehollandiae
- Black-billed Gull, Chroicocephalus bulleri
- Andean Gull, Chroicocephalus serranus
- Brown-hooded Gull, Chroicocephalus maculipennis
- Gray Gull, Leucophaeus modestus
- Dolphin Gull, Leucophaeus scoresbii
- Laughing Gull, Atricilla atricilla
- Franklin's Gull, Atricilla pipixcan
- Lava Gull, Atricilla fuliginosa
- Pallas's Gull, Ichthyaetus ichthyaetus
- Relict Gull, Ichthyaetus relictus
- Mediterranean Gull, Ichthyaetus melanocephalus
- Audouin's Gull, Ichthyaetus audouinii
- White-eyed Gull, Ichthyaetus leucophthalmus
- Sooty Gull, Ichthyaetus hemprichii
- Pacific Gull, Gabianus pacificus
- Belcher's Gull, Gabianus belcheri
- Black-tailed Gull, Gabianus crassirostris
- Olrog's Gull, Gabianus atlanticus
- Heermann's Gull, Larus heermanni
- Ring-billed Gull, Larus delawarensis
- Common Gull, Larus canus
- Short-billed Gull, Larus brachyrhynchus
- Western Gull, Larus occidentalis
- Yellow-footed Gull, Larus livens
- Kelp Gull, Larus dominicanus
- Glaucous Gull, Larus hyperboreus
- European Herring Gull, Larus argentatus
- Caspian Gull, Larus cachinnans
- Lesser Black-backed Gull, Larus fuscus
- Heuglin's Gull, Larus heuglini
- Great Black-backed Gull, Larus marinus
- Yellow-legged Gull, Larus michahellis
- Armenian Gull, Larus armenicus
- California Gull, Larus californicus
- American Herring Gull, Larus smithsonianus
- Glaucous-winged Gull, Larus glaucescens
- Iceland Gull, Larus glaucoides
- Mongolian Gull, Larus mongolicus
- Vega Gull, Larus vegae
- Slaty-backed Gull, Larus schistisagus